Answered step by step
Verified Expert Solution
Question
1 Approved Answer
begin{tabular}{|l|l|l|} hline multicolumn{1}{|c|}{IngredientFormulafor100gfinalproduct} & multicolumn{1}{|c|}{ Required Antweigh} hline Mineral Oila & 18.8g & hline Cetyl Alcohol & 5.0g & hline Arlacel 60
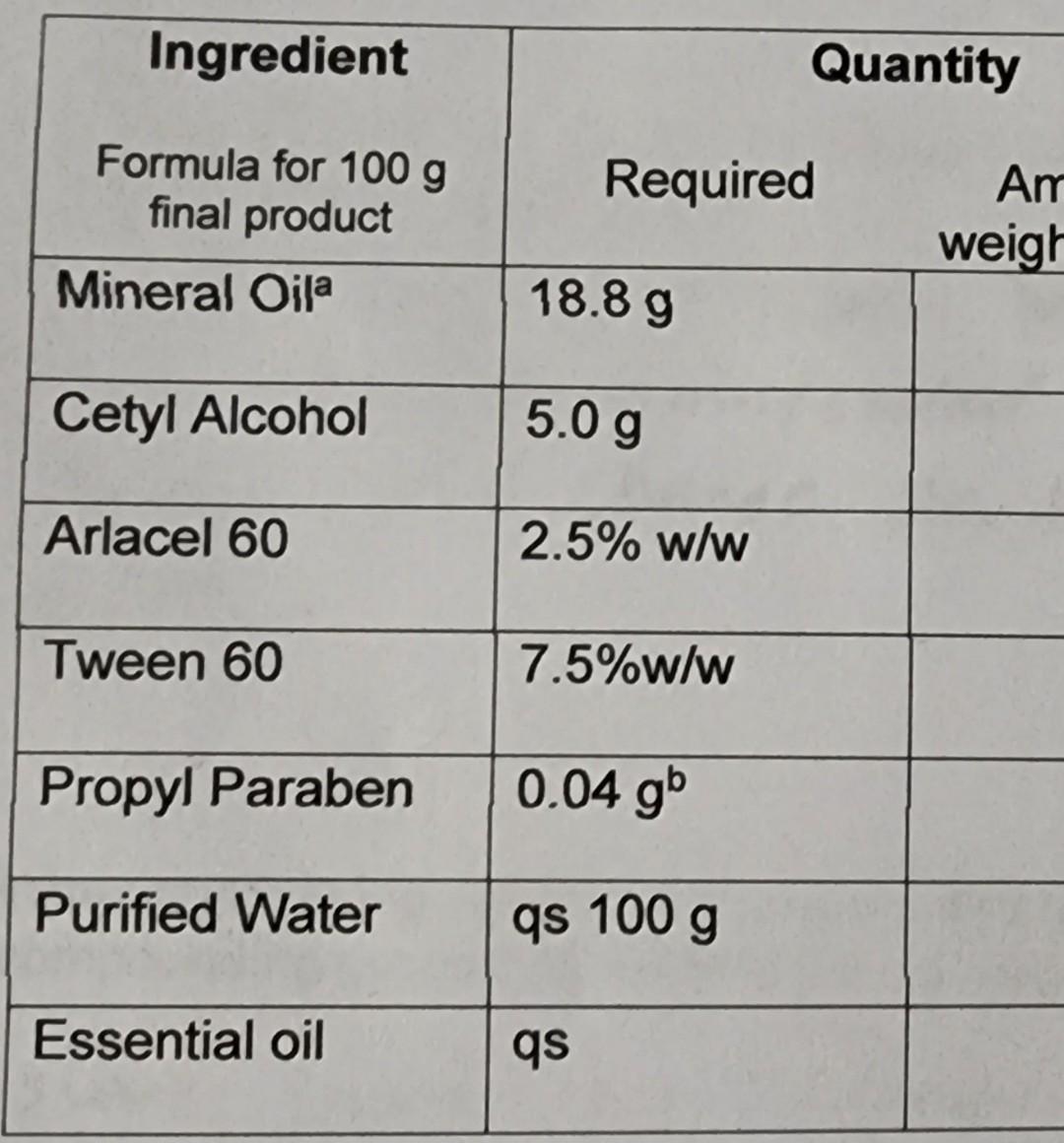


\begin{tabular}{|l|l|l|} \hline \multicolumn{1}{|c|}{IngredientFormulafor100gfinalproduct} & \multicolumn{1}{|c|}{ Required Antweigh} \\ \hline Mineral Oila & 18.8g & \\ \hline Cetyl Alcohol & 5.0g & \\ \hline Arlacel 60 & 2.5%w/w & \\ \hline Tween 60 & 7.5%w/w & \\ \hline Propyl Paraben & 0.04gb & \\ \hline Purified Water & qs 100g & \\ \hline Essential oil & qs & \\ \hline \end{tabular} aSpecific gravity of heavy mineral oil is 0.88 bYou will have a 5% w/v stock solution available, and a syringe appropriate for measuring the amount needed. *Arlacel 60 (Span 60) is also called sorbitan monostearate. It is oil soluble, not water soluble. Alcohol solubility is 50mg/mL (Source: SigmaAldrich product specifications). *Tween 60 (Polysorbate 60 ) is also called Polyoxyethylene (20) sorbitan monosteate. It is soluble in water and has a specific gravity of 1.07. (Source: ICI Americas MSDS). The solubility of propyl paraben is 1g/2500mL of water or 1.5mL alcohol. 6. Why is the combination of Arlacel 60 and Tween 60 used in this formulation
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


