Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Beneral Be the expressions for p, r, and S? 2.7 Consider a mixture of various chemical species. Define the mixture enthalpy by h = Emihy,
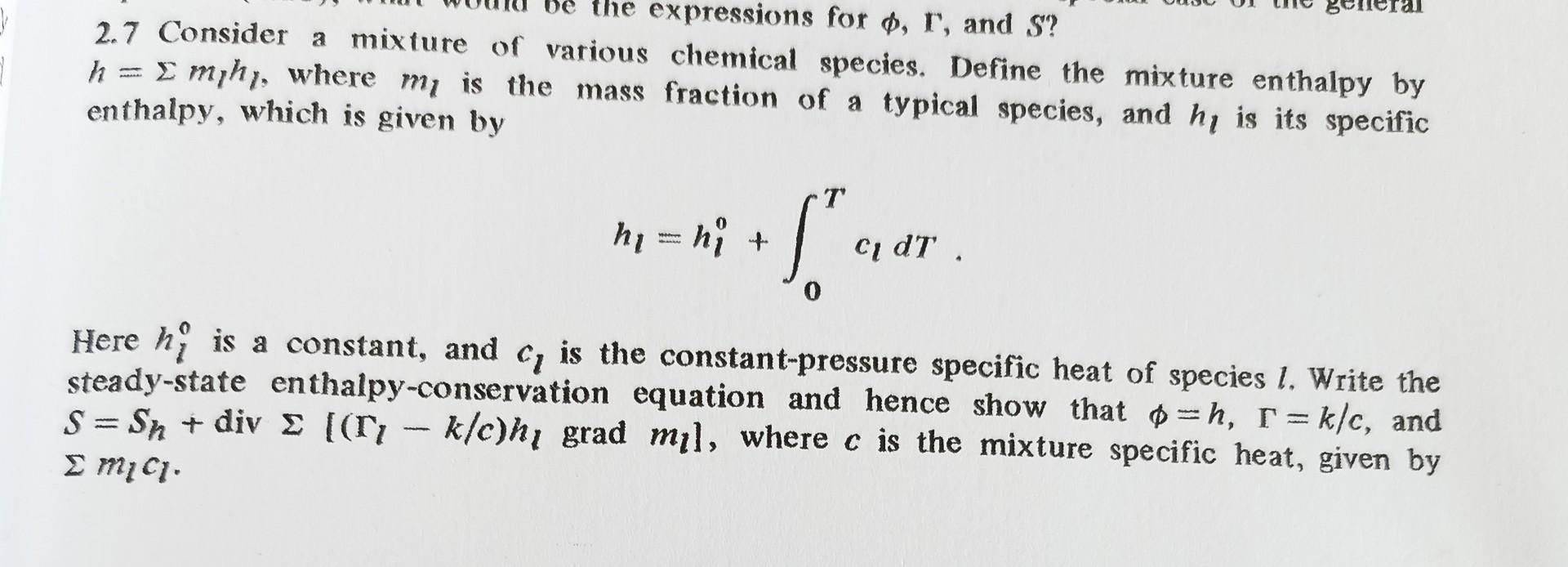
Beneral Be the expressions for p, r, and S? 2.7 Consider a mixture of various chemical species. Define the mixture enthalpy by h = Emihy, where my is the mass fraction of a typical species, and hy is its specific enthalpy, which is given by T hy=hi + $ ci d , 0 Here hi is a constant, and c, is the constant-pressure specific heat of species 1. Write the steady-state enthalpy-conservation equation and hence show that p=h, r = k/c, and S=Sn + div ((r, klc)h, grad mil, where c is the mixture specific heat, given by smici. Beneral Be the expressions for p, r, and S? 2.7 Consider a mixture of various chemical species. Define the mixture enthalpy by h = Emihy, where my is the mass fraction of a typical species, and hy is its specific enthalpy, which is given by T hy=hi + $ ci d , 0 Here hi is a constant, and c, is the constant-pressure specific heat of species 1. Write the steady-state enthalpy-conservation equation and hence show that p=h, r = k/c, and S=Sn + div ((r, klc)h, grad mil, where c is the mixture specific heat, given by smici
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


