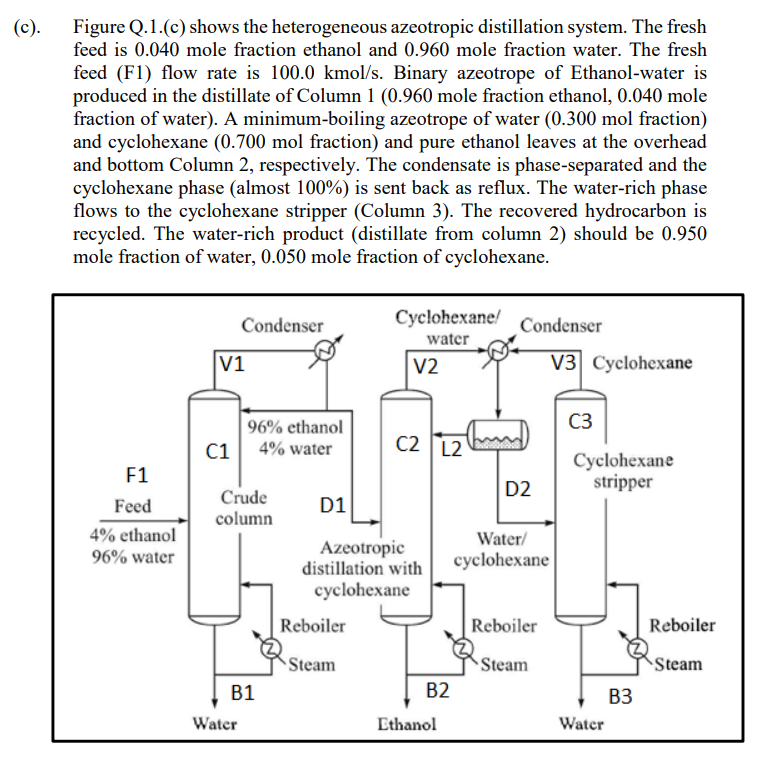
(c). Figure Q.1.(c) shows the heterogeneous azeotropic distillation system. The fresh feed is 0.040 mole fraction ethanol and 0.960 mole fraction water. The fresh feed (F1) flow rate is 100.0 kmol/s. Binary azeotrope of Ethanol-water is produced in the distillate of Column 1 (0.960 mole fraction ethanol, 0.040 mole fraction of water). A minimum-boiling azeotrope of water (0.300 mol fraction) and cyclohexane (0.700 mol fraction) and pure ethanol leaves at the overhead and bottom Column 2, respectively. The condensate is phase-separated and the cyclohexane phase (almost 100%) is sent back as reflux. The water-rich phase flows to the cyclohexane stripper (Column 3). The recovered hydrocarbon is recycled. The water-rich product (distillate from column 2) should be 0.950 mole fraction of water, 0.050 mole fraction of cyclohexane. Condenser Cyclohexane! water V2 Condenser V3 Cyclohexane V1 96% ethanol 4% water C1 C2 L2 Cyclohexane stripper D2 F1 Feed 4% ethanol 96% water Crude column D1 Water/ cyclohexane Azeotropic distillation with cyclohexane Reboiler Reboiler Reboiler Steam Steam Steam B2 B1 Water B3 Water Ethanol (c). Figure Q.1.(c) shows the heterogeneous azeotropic distillation system. The fresh feed is 0.040 mole fraction ethanol and 0.960 mole fraction water. The fresh feed (F1) flow rate is 100.0 kmol/s. Binary azeotrope of Ethanol-water is produced in the distillate of Column 1 (0.960 mole fraction ethanol, 0.040 mole fraction of water). A minimum-boiling azeotrope of water (0.300 mol fraction) and cyclohexane (0.700 mol fraction) and pure ethanol leaves at the overhead and bottom Column 2, respectively. The condensate is phase-separated and the cyclohexane phase (almost 100%) is sent back as reflux. The water-rich phase flows to the cyclohexane stripper (Column 3). The recovered hydrocarbon is recycled. The water-rich product (distillate from column 2) should be 0.950 mole fraction of water, 0.050 mole fraction of cyclohexane. Condenser Cyclohexane! water V2 Condenser V3 Cyclohexane V1 96% ethanol 4% water C1 C2 L2 Cyclohexane stripper D2 F1 Feed 4% ethanol 96% water Crude column D1 Water/ cyclohexane Azeotropic distillation with cyclohexane Reboiler Reboiler Reboiler Steam Steam Steam B2 B1 Water B3 Water Ethanol







