Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(c) To use a generalized correlation, we will compute the residual properties in the initial and final state, and then add those to the
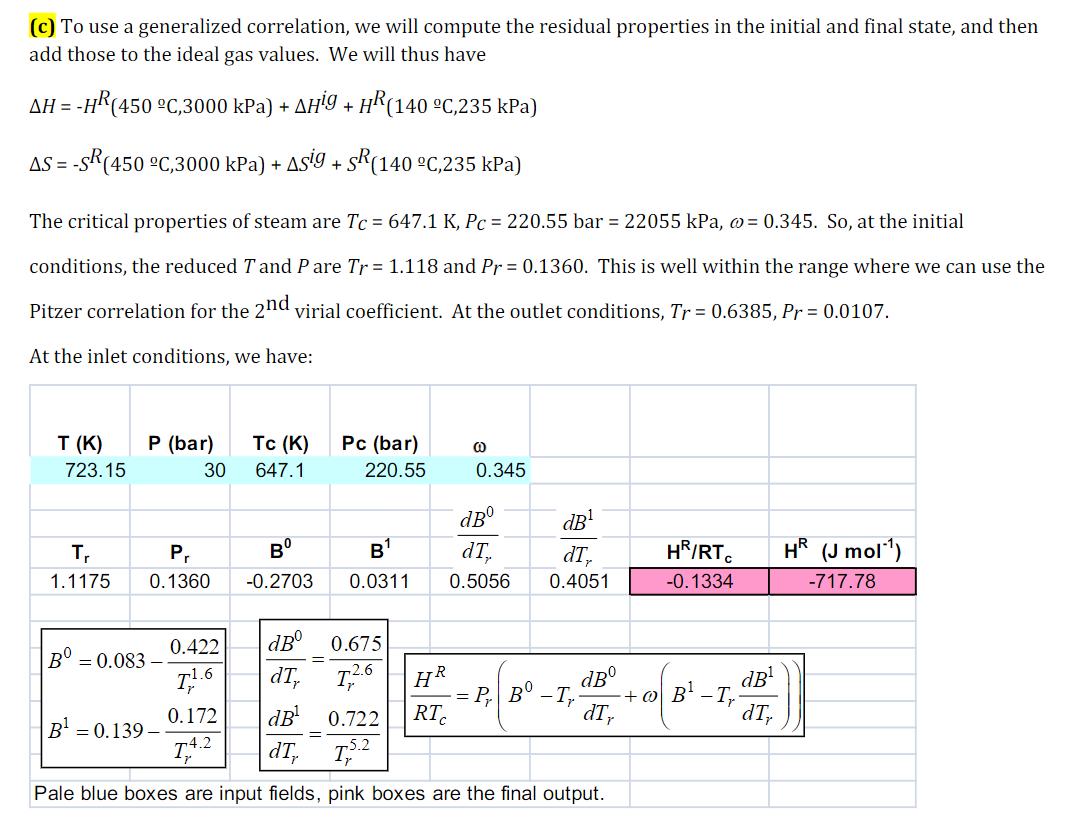
(c) To use a generalized correlation, we will compute the residual properties in the initial and final state, and then add those to the ideal gas values. We will thus have AH=-HR(450 C,3000 kPa) + AHg + HR (140 C,235 kPa) AS-SR(450 C,3000 kPa) + Asig + SR (140 C,235 kPa) The critical properties of steam are Tc = 647.1 K, Pc = 220.55 bar = 22055 kPa, = 0.345. So, at the initial conditions, the reduced T and Pare Tr = 1.118 and Pr= 0.1360. This is well within the range where we can use the Pitzer correlation for the 2nd virial coefficient. At the outlet conditions, Tr = 0.6385, Pr = 0.0107. At the inlet conditions, we have: T (K) 723.15 P (bar) 30 Tc (K) 647.1 Pc (bar) @ 220.55 0.345 dB dB T 1.1175 Pr B B dTy dTy HR/RTC HR (J mol) 0.1360 -0.2703 0.0311 0.5056 0.4051 -0.1334 -717.78 B 0.422 dB = 0.083- 11.6 dTy == 0.675 T2.6 HR P B 0.172 -T + dB dB B-T B = 0.139- dB 0.722 RTC dTy dTy = T4.2 dTy T Pale blue boxes are input fields, pink boxes are the final output.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


