Question
C5H12 (g)+ O2 (g) CO2 (g) + HO (e) AH rxn=-3535.9 kJ/mol CO2 (g) AH = -393.5 kJ/mol H2O (l) AH = -285.8 kJ/mol
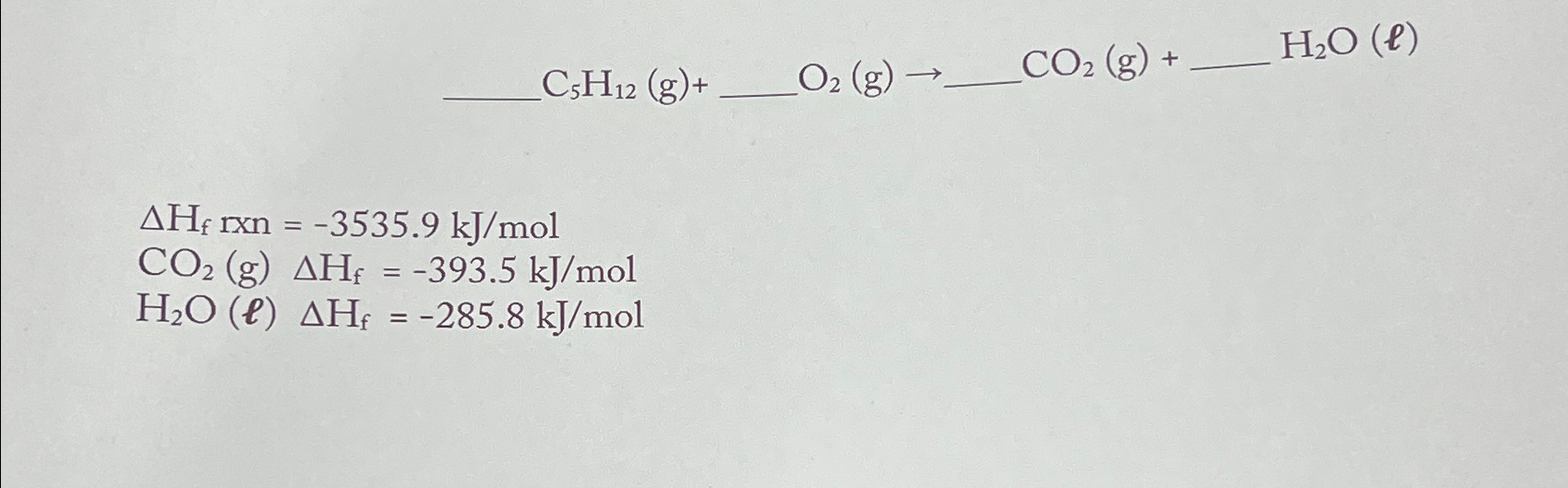
C5H12 (g)+ O2 (g) CO2 (g) + HO (e) AH rxn=-3535.9 kJ/mol CO2 (g) AH = -393.5 kJ/mol H2O (l) AH = -285.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Making Hard Decisions with decision tools
Authors: Robert Clemen, Terence Reilly
3rd edition
538797576, 978-0538797573
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



