Answered step by step
Verified Expert Solution
Question
1 Approved Answer
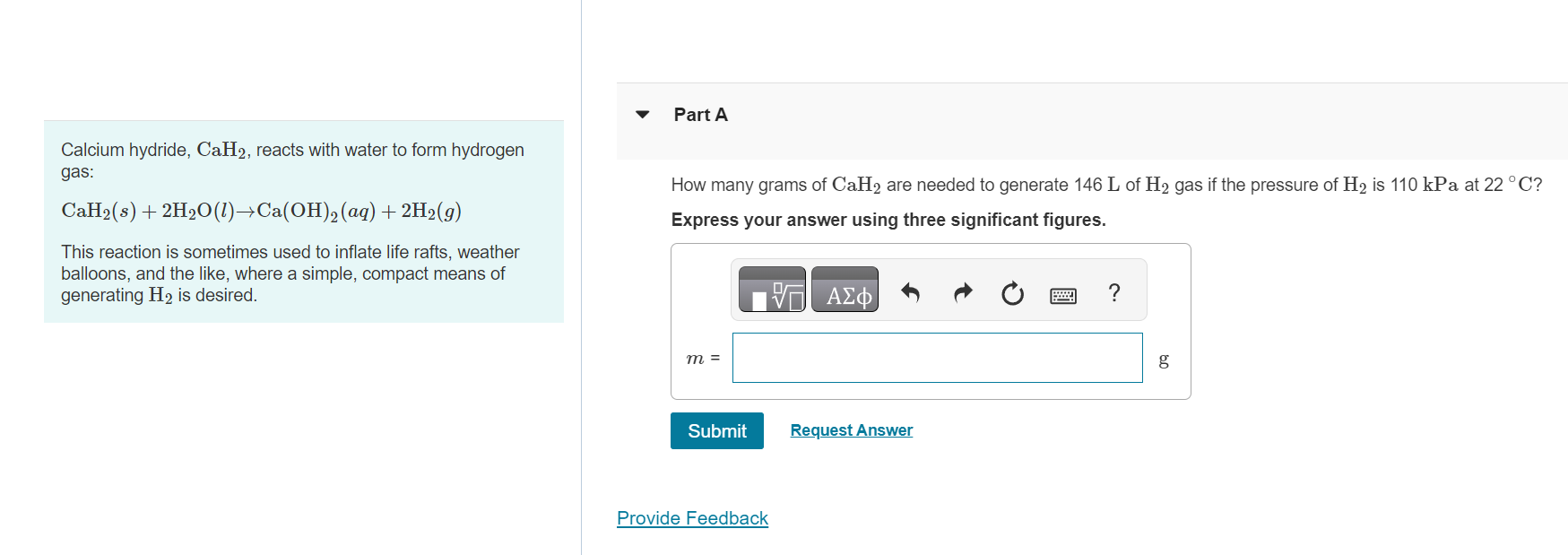
Calcium hydride, C a H 2 , reacts with water to form hydrogen gas: C a H 2 ( s ) + 2 H 2
Calcium hydride, reacts with water to form hydrogen
gas:
This reaction is sometimes used to inflate life rafts, weather
balloons, and the like, where a simple, compact means of
generating is desired.
Part A
How many grams of are needed to generate of gas if the pressure of is kPa at
Express your answer using three significant figures.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


