Answered step by step
Verified Expert Solution
Question
1 Approved Answer
calculate a. T product b. If the N gas leaving the tower absorbs into the Cooler and the temperature leaving the Cooler is 50F how
calculate
20. Diketahui skema proses sebagai berikut: NaOH 20%, 80F 1000 ftjam Menara No. 65F Cooler 50F CO20% dan absorbsi Nz 80%. 650 Tproduk = ? Na2CO3 Reaksi yang terjadi: 2 NaOH + CO2 Na2CO3 Hitung a. T produk b. Kalau gas N, yang keluar dari menara absorbs masuk ke Cooler dan suhu yang keluar dari Cooler 50F berapa panas yang diambil oleh pendingin setiap jam? 20. Diketahui skema proses sebagai berikut: NaOH 20%, 80F 1000 ftjam Menara No. 65F Cooler 50F CO20% dan absorbsi Nz 80%. 650 Tproduk = ? Na2CO3 Reaksi yang terjadi: 2 NaOH + CO2 Na2CO3 Hitung a. T produk b. Kalau gas N, yang keluar dari menara absorbs masuk ke Cooler dan suhu yang keluar dari Cooler 50F berapa panas yang diambil oleh pendingin setiap jam a. T product
b. If the N gas leaving the tower absorbs into the Cooler and the temperature leaving the Cooler is 50F how much heat is taken up by the cooler every hour? 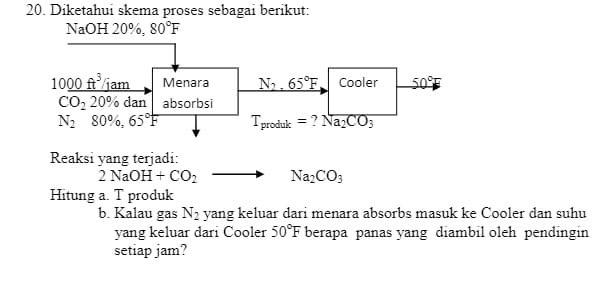

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


