Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate and enter the 'initial' molarity (M) of Fe+ and SCN, and the 'final' molar concentration of FeSCN2+ (M) present in the 25.00 mL
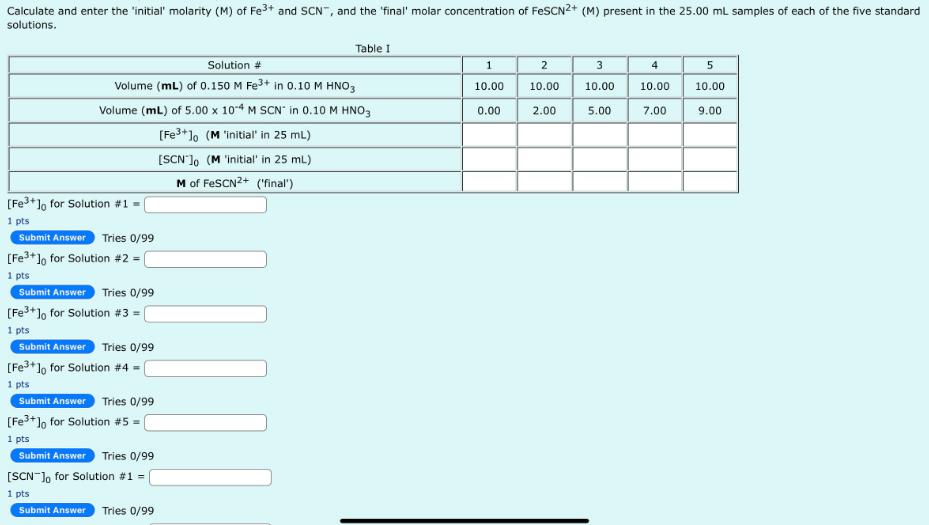
Calculate and enter the 'initial' molarity (M) of Fe+ and SCN", and the 'final' molar concentration of FeSCN2+ (M) present in the 25.00 mL samples of each of the five standard solutions. Solution # Volume (mL) of 0.150 M Fe+ in 0.10 M HNO3 Volume (mL) of 5.00 x 104 M SCN in 0.10 M HNO3 [Fe+]o (M 'initial' in 25 mL) [SCN] (M 'initial' in 25 mL) M of FeSCN2+ ('final") [Fe+] for Solution #1 1 pts Submit Answer Tries 0/99 [Fe+] for Solution #2 1 pts Submit Answer Tries 0/99 [Fe+] for Solution #3 = ( 1 pts Submit Answer Tries 0/99 [Fe+] for Solution #4 = ( 1 pts Submit Answer Tries 0/99 [Fe+] for Solution #5 1 pts Table I Submit Answer Tries 0/99 [SCN-] for Solution #1 = | 1 pts Submit Answer Tries 0/99 1 10.00 0.00 2 10.00 2.00 3 10.00 5.00 4 10.00 7.00 5 10.00 9.00
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the initial molarity of Fe3 and SCN and the final molar concentration of FeSCN2 for eac...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


