Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate heat loss to be recovered? Surface heat loss Fuel-100F 403,800 cfh 415,880,000 Btu/hr Combustion air-100F 5,250,000 cfh 6,300,000 Btu/hr Product-100F 50 tons/hr 500,000
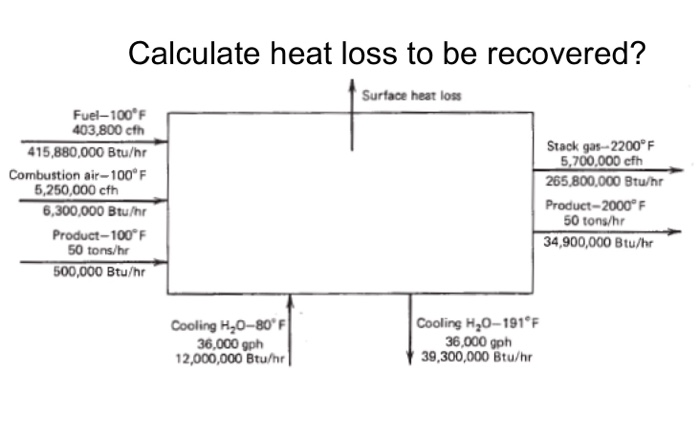
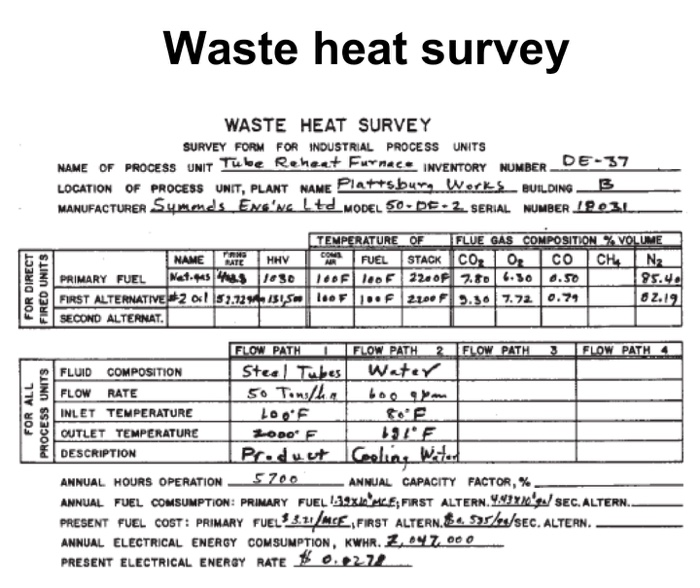
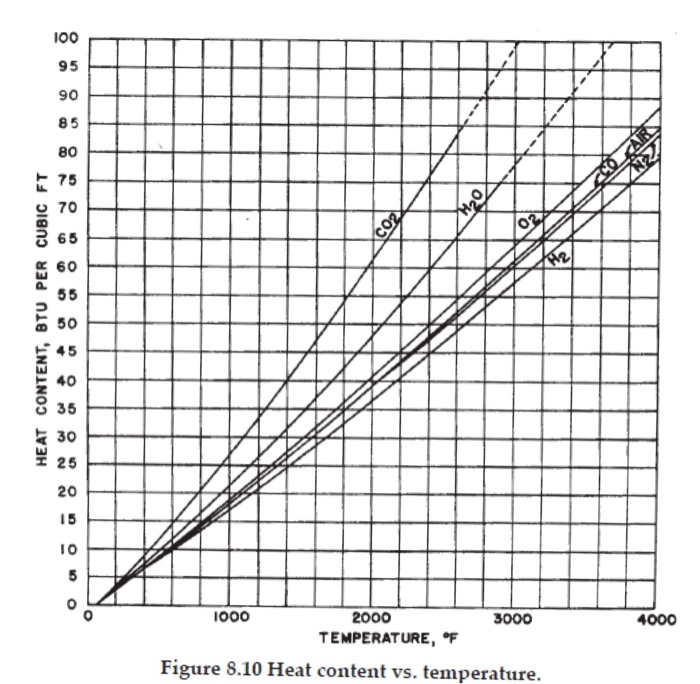
Calculate heat loss to be recovered? Surface heat loss Fuel-100F 403,800 cfh 415,880,000 Btu/hr Combustion air-100F 5,250,000 cfh 6,300,000 Btu/hr Product-100F 50 tons/hr 500,000 Btu/hr Cooling H0-80 F 36,000 gph 12,000,000 Btu/hr Cooling H0-191F 36,000 gph 39,300,000 Btu/hr Stack gas-2200F 5,700,000 cfh 265,800,000 Btu/hr Product-2000F 50 tons/hr 34,900,000 Btu/hr FOR DIRECT FIRED UNITS FOR ALL PROCESS UNITS Waste heat survey WASTE HEAT SURVEY SURVEY FORM FOR INDUSTRIAL PROCESS UNITS NAME OF PROCESS UNIT Tube Reheat Furnace INVENTORY NUMBER. DE-37 LOCATION OF PROCESS UNIT, PLANT NAME Plattsburg Works BUILDING B MANUFACTURER Symonds ENGING Ltd_MODEL 50-DE-2. SERIAL NUMBER 19031 FIRING HHV NAME RATE Net.qus 408.3 1030 PRIMARY FUEL FIRST ALTERNATIVE #2 01 52.729 131,5m SECOND ALTERNAT. FLUID COMPOSITION FLOW RATE INLET TEMPERATURE OUTLET TEMPERATURE DESCRIPTION TEMPERATURE OF FLUE GAS COMPOSITION % VOLUME AIR FUEL STACK CO 0 CH N COMS 100F 100F 2200F 7.80 6.30 0.50 25.44 100F 100F 2200 F 9.30 7.72 0.79 82.19 FLOW PATH Steel Tubes 50 Tons/h FLOW PATH 2 FLOW PATH Water LOOF 2000 F 191F Product Cooling Water 5700 3 FLOW PATH 4 ANNUAL HOURS OPERATION SEC. ALTERN.. ANNUAL CAPACITY FACTOR, % ANNUAL FUEL COMSUMPTION: PRIMARY FUEL 139XCE; FIRST ALTERN. 437 PRESENT FUEL COST: PRIMARY FUEL 3.21/MCE, FIRST ALTERN.. 595/2/SEC. ALTERN.. ANNUAL ELECTRICAL ENERGY COMSUMPTION, KWHR. 2,047, 000 PRESENT ELECTRICAL ENERGY RATE 0.0278 HEAT CONTENT, BTU PER CUBIC FT 100 95 90 85 80 75 70 65 60 55 50 40 35 30 25 95 20 15 10 5 CO 1000 H0 0 2000 TEMPERATURE, OF Figure 8.10 Heat content vs. temperature. 3000 1 CAIR 4000
Step by Step Solution
★★★★★
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
We must add up the heat losses from each component to determine the overall amount of heat loss that ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


