Question
Calculate the half-cell potential of Ni in 0.1M NiCl2. The half-cell is represented as: NiNi2+, Cl- (0.1M). Cation Anion Ag+ +77.1 Br -102.8 Al3+ -483.1
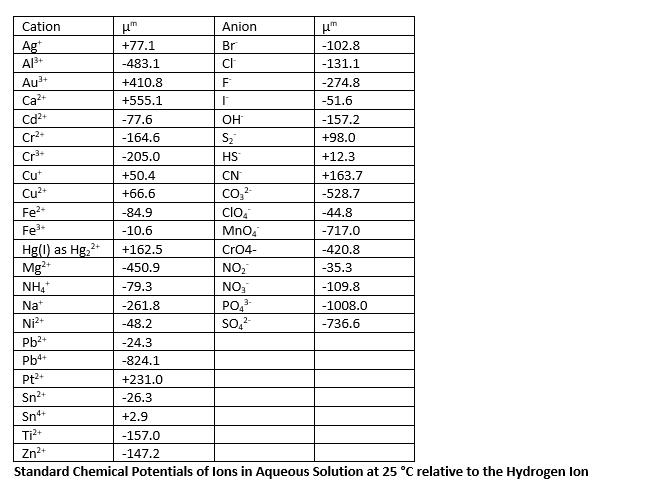
Cation Anion Ag+ +77.1 Br -102.8 Al3+ -483.1 cl -131.1 Au+ +410.8 F -274.8 Ca2+ +555.1 -51.6 Cd2+ -77.6 OH -157.2 Cr+ -164.6 S +98.0 Cr+ -205.0 HS +12.3 Cu+ +50.4 CN +163.7 Cu2+ +66.6 -528.7 Fe- -84.9 CIOA -44.8 Fe3+ -10.6 MnO4 -717.0 Hg(1) as Hg22+ +162.5 CrO4- -420.8 Mg2+ -450.9 NO -35.3 NH4+ -79.3 NO3 -109.8 Na+ -261.8 PO43 -1008.0 Ni+ -48.2 SO -736.6 Pb+ -24.3 Pb4+ -824.1 Pt2+ +231.0 Sn+ -26.3 Sn4+ +2.9 Ti2+ -157.0 Zn2+ -147.2 Standard Chemical Potentials of lons in Aqueous Solution at 25 C relative to the Hydrogen lon
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the halfcell potential of Ni in 01M NiCl2 we need to use the Nernst equation E E 00592n ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



