Calculate the number of cations, anions, and formula units in a unit cell of each of the
Question:
Calculate the number of cations, anions, and formula units in a unit cell of each of the following solids:
(a) The cesiumchloride unit cell shown in Fig. 3H.30;
(b) The rutile (TiO2) unit cell shown here.
(c) What are the coordination numbers of the ions in rutile?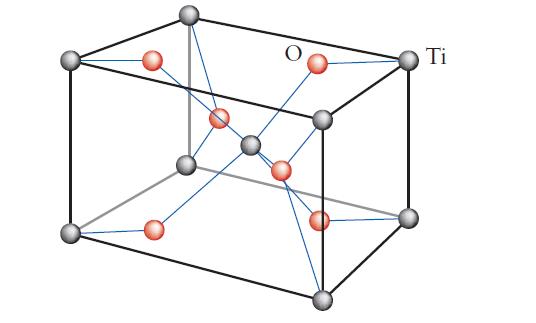
FIGURE 3H.30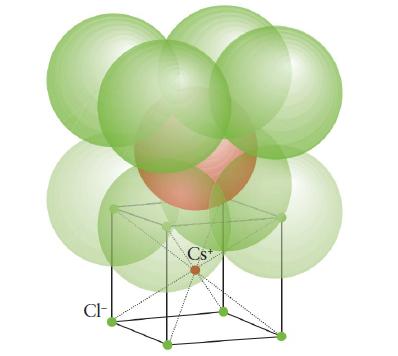
Transcribed Image Text:
Ti
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a One Cs and one Cl in each unit cell and one fo...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Java understanding: What this code is and how it works What does the while(x < n) mean then try and catch explain read.useDelimiter(" "); //returns the scanner object/setting the pattern int x = 0;...
-
For the following two graphics, provide the specified information below for each. Inverse Demand: P= 43.75 - .00625 Q; MR = 43.75 - 0.0125 Q 25 20 15 $ per unit 10 10 5 0 MC 500 1000 1500 ATC 2000 -...
-
Calculate the number of cations and anions in each of the following compounds: (a) 8.38 g of KBr, (b) 5.40 g of Na2 SO4, (c) 7.45 g of Ca3 (PO4)2.
-
5 Melbourne Corporation has traditionally made a subcomponent of its major product. Annual production of 30,000 subcomponents results in the following costs: Direct materials Direct labor Variable...
-
If the magnitude of the couple moment acting on the pipe assembly is 50 N ? m, determine the magnitude of the couple forces applied to each wrench. The pipe assembly lies in the x?y plane. 300 mm 300...
-
Refer to Exercise 24. Suppose each cars price is reduced by 10% instead of $500. What effect will this discount have on each of the following characteristics of the resulting price distribution? a....
-
20. Firm A has a stock price of \($40\) and has made an offer for firm B where A promises to pay $60/share for B, as long as As stock price remains between \($35\) and \($45\). If the price of A is...
-
The water clock (clepsydra) shown in Fig. P3.101 is an ancient device for measuring time by the falling water level in a large glass container. The water slowly drains out through a small hole in the...
-
Old MathJax webview comparing three depreciation methods Comparing Three Depreciation Methods Waylander Coatings Company purchased waterproofing equipment on January 6 for $320,000. The equipment was...
-
The air in the space suit of astronauts is actually pure oxygen supplied at a pressure of 0.30 bar. Each of the two tanks on a space suit has a volume of 3980. cm 3 and an initial pressure of 5860....
-
When long surfactant molecules having a polar headgroup and a nonpolar tail are added to water, micelles are formed in which the nonpolar tails aggregate, with the polar headgroups pointing out...
-
Design a collection of database tables for the Springfield Town Council. The council is made up of representatives from each of the towns 15 precincts. The data you need to store includes the...
-
Avery, an unmarried taxpayer, had the following income items: Salary Net income from a rental house 3 7 , 0 5 0 4 , 9 0 0 Avery has a 4 - year - old child who attends a child care center. Assume the...
-
California Lottery Let A denote the event of placing a $1 straight bet on the California Daily 4 lottery and winning. There are 10,000 different ways that you can select the four digits (with...
-
"Tamara Wiley glanced in the mirror before leaving her apartment and heading to her 8 a.m. class. She was having a bad hair day, so she had thrown on a scarf. Her quick check in the mirror told her...
-
Online Friends In a Pew Research Center survey of 1060 teens aged 13 to 17, it was found that 604 (or 57.0%) of those respondents have made new friends online. If the true rate is 50%, there is a...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
The graphs of polar equations in the forms r = a(cos + 1), r = a(cos - 1), r = a(sin + 1), and r = a(sin - 1) are called cardioids because they resemble hearts. Graph several curves in the...
-
A report from the college dean indicates that for the previous semester, the grade distribution for the Department of Psychology included 135 As, 158 Bs, 140 Cs, 94 Ds, and 53 Fs. Determine what kind...
-
A galvanic cell is based on the following half reactions: Cu 2+ + 2e - Cu(s) o = 0.34 V V 2+ + 2e - V(s) o = 21.20 V In this cell the copper compartment contains a copper electrode and [Cu 2+ ] =...
-
Answer the following questions using data from Table (all under standard conditions). a. Is H + (aq) capable of oxidizing Cu(s) to Cu 2+ (aq)? b. Is Fe 3+ (aq) capable of oxidizing I 2 (aq)? c. Is H...
-
Using data from Table, place the following in order of increasing strength as oxidizing agents (all under standard conditions). Cd 2+ , IO 3 - , K1, H 2 O, AuCl 4 - , and I 2 Table Standard Reduction...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


