Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the molar mass of CO2 assuming a temperature of 273.0K. Tries 6/99 Previous Tries Calculate the Gas constant, R, in Latm/Kmol. Tries 1/99 Previous
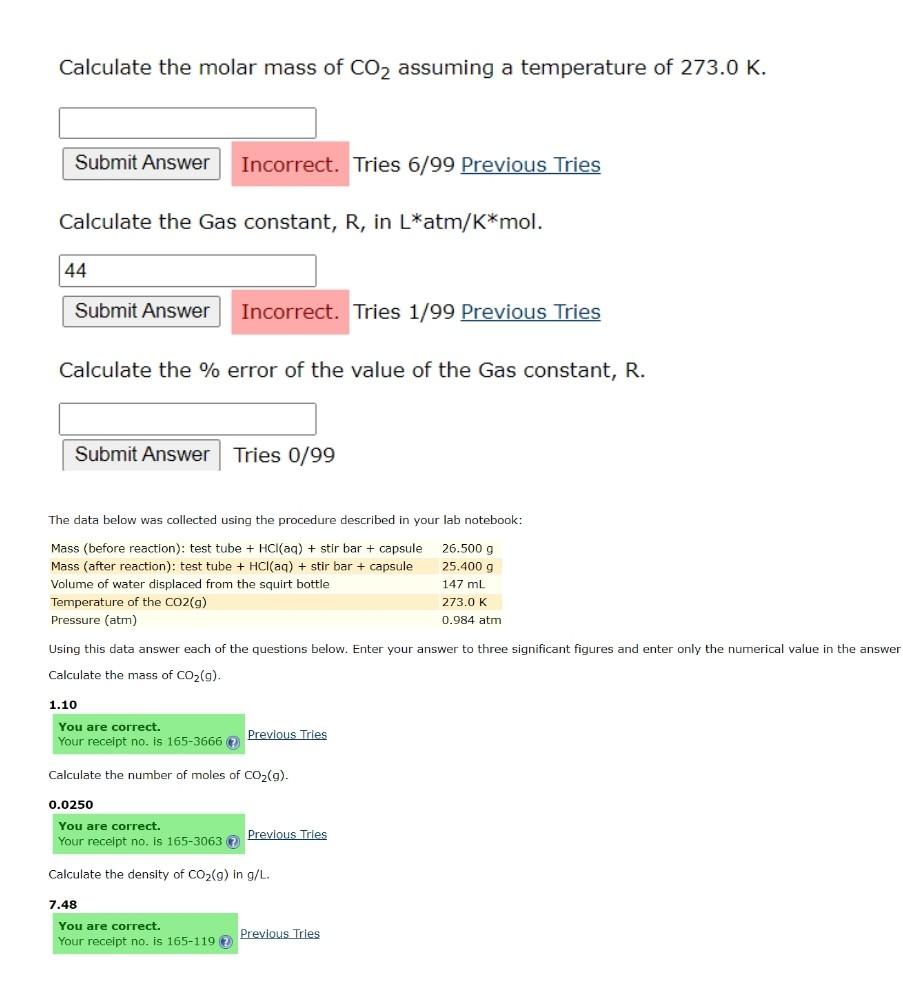
Calculate the molar mass of CO2 assuming a temperature of 273.0K. Tries 6/99 Previous Tries Calculate the Gas constant, R, in Latm/Kmol. Tries 1/99 Previous Tries Calculate the \% error of the value of the Gas constant, R. Tries 0/99 The data below was collected using the procedure described in your lab notebook: Using this data answer each of the questions below. Enter your answer to three significant figures and enter only the numerical value in the answer Calculate the mass of CO2(g). 1.10 Calculate the number of moles of CO2(g). 0.0250 Previous Tries Calculate the density of CO2(g) in g/L. 7.48
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


