Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the standard cell potential for each of the following electrochemical cells. You may want to reference (Pages 854-861) Section 19.4 while completing this
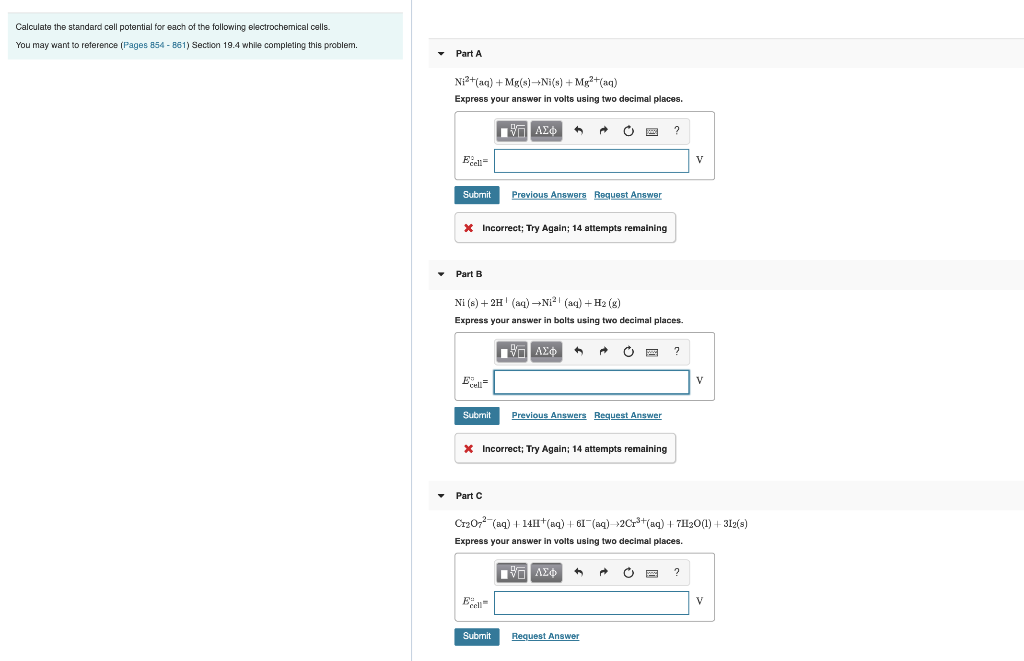
Calculate the standard cell potential for each of the following electrochemical cells. You may want to reference (Pages 854-861) Section 19.4 while completing this problem. Part A Ni+ (aq) + Mg(s)Ni(s) +Mg+(aq) Express your answer in volts using two decimal places. Excell Submit Part B * Incorrect; Try Again; 14 attempts remaining Ecell Submit 17 Ni (s) + 2H (aq) N (aq) + H(g) Express your answer in bolts using two decimal places. Part C Previous Answers Request Answer Ecell Submit |-- VO * Incorrect; Try Again; 14 attempts remaining ^ Previous Answers Request Answer ? CrO7 (aq) + 14H+ (aq) + 61(aq)2Cr+ (aq) +7HO(1)+312(s) Express your answer in volts using two decimal places. [35] Request Answer ? V 2 V V
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Answer Ans A Ni aq Mgs E cell Cathode Ni s Mg 2 aq An...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


