Calculate the standard Gibbs free energy change for the following ethanol decomposition reaction at 443K:
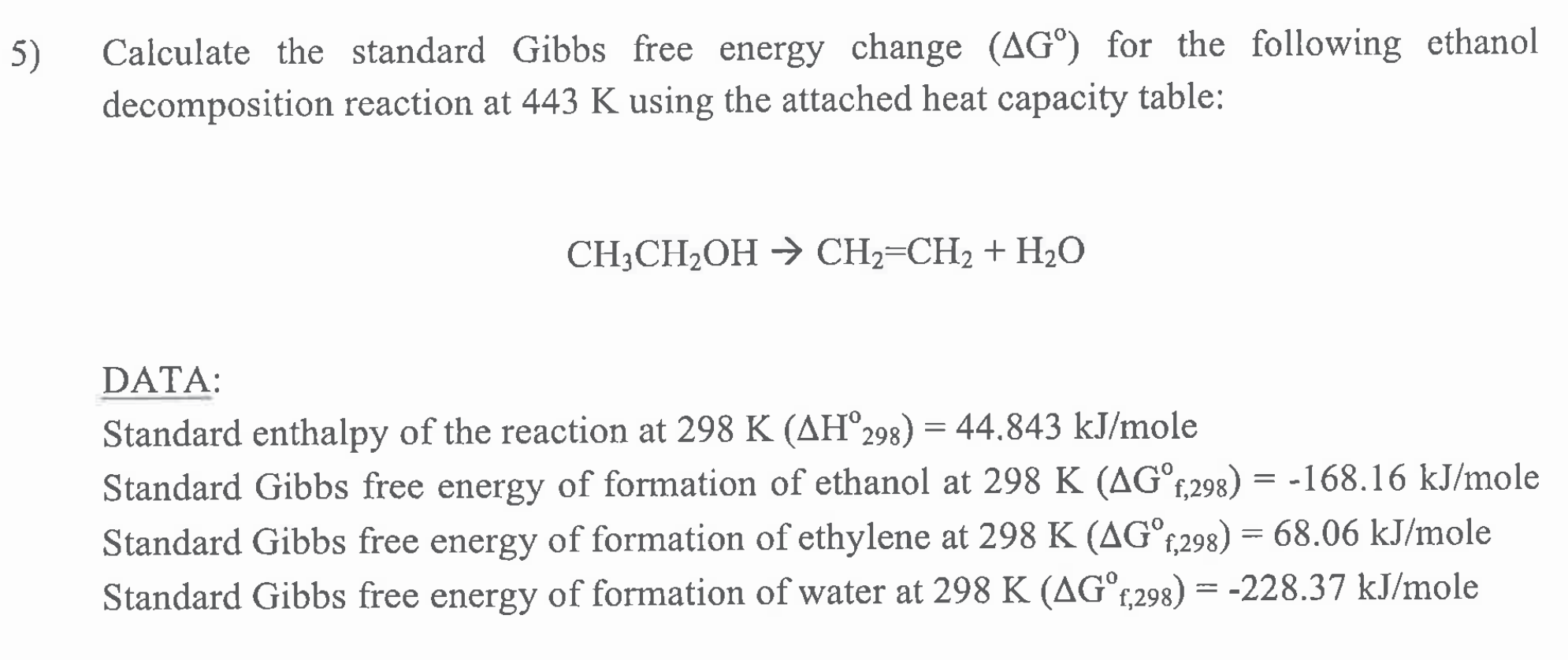
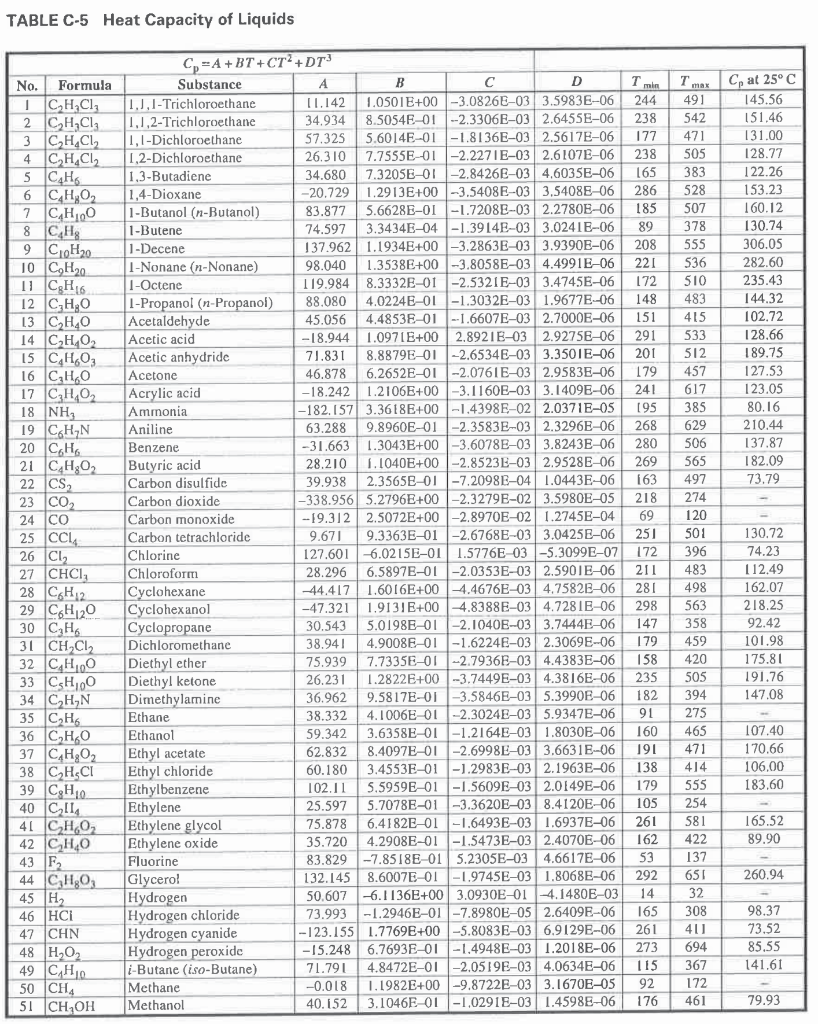
5) Calculate the standard Gibbs free energy change (AG) for the following ethanol decomposition reaction at 443 K using the attached heat capacity table: CH3CH2OH CH2=CH2 + H2O - = DATA: Standard enthalpy of the reaction at 298 K (AH298) = 44.843 kJ/mole Standard Gibbs free energy of formation of ethanol at 298 K (AGf,298) = -168.16 kJ/mole Standard Gibbs free energy of formation of ethylene at 298 K (AG1,298) = 68.06 kJ/mole Standard Gibbs free energy of formation of water at 298 K (AGc,298) = -228.37 kJ/mole = = TABLE C-5 Heat Capacity of Liquids Cp at 25C 145.56 151.46 131.00 128.77 122.26 153.23 160.12 130.74 306.05 282.60 235.43 144.32 102.72 128.66 189.75 127.53 123.05 80.16 210.44 137.87 182.09 73.79 No. Formula 1 C2H4Cl 2 CH2Cl2 3 CH.CL 4 C, H.CL 5 CAH 6 C,H,O2 7 CH10 8 CH 9 CloH20 10 C,H20 11 C H 6 12 C,H,O 13 CHO 14 CHO 15 C,H,O, 16 C H20 17 CHO 18 NH 19 C H N 20 CH 21 C H202 22 CS 23 CO2 24 CO 25 cc, 26 CL 27 CHCI 28 CH2 29 C6H120 30 CH 31 CH.CL2 32 C H100 33 C,H,00 34 CHUN 35 CH 36 CHO 37 C,H,O2 38 |CH,CI 39 C Ho. 40 CM 4 CH 02 42 C2H40 43 F 44 C,H,O, 45 H 46 HCI 47 CHN 48 H202 49 |CAH10 50 CHA 51 CH OH C=A+BT+CT? +D13 Substance A B D 1.1.1- Trichloroethane 11.142 1.0501E+00 -3.0826E-03 3.5983E-06 1.1.2-Trichloroethane 34.934 8.5054E-01 -2.33060-03 2.6455E-06 1,1-Dichloroethane 57.325 5.6014E-01 -1.8136E-03 2.5617E-06 1,2-Dichloroethane 26.310 7.7555E-01 -2.2271E-03 2.6107E-06 1,3-Butadiene 34.680 7.320SE-01 -2.8426E-03 4.6035E-06 1,4-Dioxane -20.729 1.2913E+00 -3.5408E-03 3,54086-06 1-Butanol (n-Butanol) 83.877 5.6628E-01 -1.7208E-03 2.2780E-06 1-Butene 74.597 3.3434E-04 -1.3914E-03 3.0241E-06 1-Decene 137.962 1.1934E+00 -3.2863E-03 3.93908-06 1-Nonane (n-Nonane) 98.040 1.3538E+00 -3.8058E-03 4.4991E-06 1-Octene 119.9848.3332E-01 -2.5321E-03 3.4745E-06 1-Propanol (n-Propanol) 88,080 4.0224E-01 -1.3032E-03 1.9677E-06 Acetaldehyde 45.056 4.4853E-01 --1.6607E-03 2.7000E-06 Acetic acid -18.944 1.0971E+00 2.8921B-03 2.92758-06 Acetic anhydride 71.831 8.8879E-01 -2.6534E-03 3.3501E-06 Acetone 46.878 6.2652E-01 -2.0761E-032.95830-06 Acrylic acid -18.242 1.2106E+00 -3.1160E-03 3.1409E-06 Ammonia -182.157 3.3618E+00 -1.4398E-02 2.0371E-05 Aniline 63.288 9.8960E-01 -2.3583E-03 2.3296E-06 Benzene -31.663 1.3043E+00 -3.6078E-03 3.8243E-06 Butyric acid 28.210 1.1040E+00 -2.8523E-03 2.9528E-06 Carbon disulfidc 39.938 2.35658-01-7.20986-04 1.0443E-06 Carbon dioxide -338.956 5.2796E+00 -2.3279E-02 3.5980E-05 Carbon monoxide - 19.3122.5072E+00 -2.8970E-02 1.2745E-04 Carbon tetrachloride 9.671 9.3363E-01 -2.6768E-03 3.04250-06 Chlorine 127.601 6.0215E-01 1.5776E-03 -5.30990-07 Chloroform 28.296 6.58970-01 -2.0353E-03 2.5901E-06 Cyclohexane -44.417 1.6016E+00 -4.4676E-03 4.75828-06 Cyclohexanol -47.321 1.9131E+00 -4.8388E-03 4.7281E-06 Cyclopropane 30.543 5.0198E-01 -2.10406-03 3.7444E-06 Dichloromethane 38.941 4.90086-01-16224E-03 2.3069E-06 Diethyl ether 75.939 7.7335E-01 -2.7936E-03 4.4383E-06 Diethyl ketone 26.231 1.2822E+00 -3.7449E-03 4.3816E-06 Dimethylamine 36.962 9.5817E-01 -3.5846E-03 5.39906-06 Ethane 38.332 4.1006E 01 -2.3024E-03 5.9347E-06 Ethanol 59.342 3.6358E-01 -1.2164E-03 1.8030E-06 Ethyl acetate 62.832 8.4097E-01 -2.6998E-03 3.6631E-06 Ethyl chloride 60.180 3.4553E-01 -1.2983E-03 2.1963E-06 Ethylbenzene 102.11 5.5959E-01 -1.5609E-03 2.0149E-06 Ethylene 25.597 5.7078E-01 -3.3620E-03 8.4120E-06 Ethylene glycol 75.878 6.4182E-01 -1.6493E-03 1.6937E-06 Ethylene oxide 35.720 4.2908E-01 -1.5473E-032.4070E-06 Fluorine 83.829 -7.8518E-01 5.2305E-03 4.6617E-06 Glycerol 132.145 8.6007E-01 -1.9745E-03 1.8068E-06 Hydrogen 50.607 -6.1136E+00 3.0930E-01 -4.1480E-03 Hydrogen chloride 73.993 -1.2946E-01 -7.8980E-05 2.6409E-06 Hydrogen cyanide -123.155 1.7769E+00 -5.8083E-03 6.9129E-06 Hydrogen peroxide -15.248 6.7693E-01 -1.4948E-03 1.2018E-06 i-Butane (iso-Butane) 71.791 4.8472E-01 -2.0519E-03 4.06348-06 Methane -0.018 1.1982E+00 -9.8722E-03 3.1670E-05 Methanol 40.152 3.1046E-01-1,0291E-03 1.45988-06 T min 244 238 177 238 165 286 185 89 208 221 172 148 151 291 201 179 241 195 268 280 269 163 218 69 251 172 211 281 298 147 179 158 235 182 91 160 191 138 179 105 261 162 53 292 14 165 261 273 115 92 176 Tmx 491 542 471 505 383 528 507 378 555 536 510 483 415 533 512 457 617 385 629 506 565 497 274 120 501 396 483 498 563 358 459 420 505 394 275 465 471 414 555 254 581 422 137 651 32 308 40 694 367 172 461 130.72 74.23 112.49 162.07 218.25 92.42 101.98 175.81 191.76 147,08 107.40 170.66 106,00 183.60 165.52 89.90 98.37 73.52 85.55 141.61 79.93 5) Calculate the standard Gibbs free energy change (AG) for the following ethanol decomposition reaction at 443 K using the attached heat capacity table: CH3CH2OH CH2=CH2 + H2O - = DATA: Standard enthalpy of the reaction at 298 K (AH298) = 44.843 kJ/mole Standard Gibbs free energy of formation of ethanol at 298 K (AGf,298) = -168.16 kJ/mole Standard Gibbs free energy of formation of ethylene at 298 K (AG1,298) = 68.06 kJ/mole Standard Gibbs free energy of formation of water at 298 K (AGc,298) = -228.37 kJ/mole = = TABLE C-5 Heat Capacity of Liquids Cp at 25C 145.56 151.46 131.00 128.77 122.26 153.23 160.12 130.74 306.05 282.60 235.43 144.32 102.72 128.66 189.75 127.53 123.05 80.16 210.44 137.87 182.09 73.79 No. Formula 1 C2H4Cl 2 CH2Cl2 3 CH.CL 4 C, H.CL 5 CAH 6 C,H,O2 7 CH10 8 CH 9 CloH20 10 C,H20 11 C H 6 12 C,H,O 13 CHO 14 CHO 15 C,H,O, 16 C H20 17 CHO 18 NH 19 C H N 20 CH 21 C H202 22 CS 23 CO2 24 CO 25 cc, 26 CL 27 CHCI 28 CH2 29 C6H120 30 CH 31 CH.CL2 32 C H100 33 C,H,00 34 CHUN 35 CH 36 CHO 37 C,H,O2 38 |CH,CI 39 C Ho. 40 CM 4 CH 02 42 C2H40 43 F 44 C,H,O, 45 H 46 HCI 47 CHN 48 H202 49 |CAH10 50 CHA 51 CH OH C=A+BT+CT? +D13 Substance A B D 1.1.1- Trichloroethane 11.142 1.0501E+00 -3.0826E-03 3.5983E-06 1.1.2-Trichloroethane 34.934 8.5054E-01 -2.33060-03 2.6455E-06 1,1-Dichloroethane 57.325 5.6014E-01 -1.8136E-03 2.5617E-06 1,2-Dichloroethane 26.310 7.7555E-01 -2.2271E-03 2.6107E-06 1,3-Butadiene 34.680 7.320SE-01 -2.8426E-03 4.6035E-06 1,4-Dioxane -20.729 1.2913E+00 -3.5408E-03 3,54086-06 1-Butanol (n-Butanol) 83.877 5.6628E-01 -1.7208E-03 2.2780E-06 1-Butene 74.597 3.3434E-04 -1.3914E-03 3.0241E-06 1-Decene 137.962 1.1934E+00 -3.2863E-03 3.93908-06 1-Nonane (n-Nonane) 98.040 1.3538E+00 -3.8058E-03 4.4991E-06 1-Octene 119.9848.3332E-01 -2.5321E-03 3.4745E-06 1-Propanol (n-Propanol) 88,080 4.0224E-01 -1.3032E-03 1.9677E-06 Acetaldehyde 45.056 4.4853E-01 --1.6607E-03 2.7000E-06 Acetic acid -18.944 1.0971E+00 2.8921B-03 2.92758-06 Acetic anhydride 71.831 8.8879E-01 -2.6534E-03 3.3501E-06 Acetone 46.878 6.2652E-01 -2.0761E-032.95830-06 Acrylic acid -18.242 1.2106E+00 -3.1160E-03 3.1409E-06 Ammonia -182.157 3.3618E+00 -1.4398E-02 2.0371E-05 Aniline 63.288 9.8960E-01 -2.3583E-03 2.3296E-06 Benzene -31.663 1.3043E+00 -3.6078E-03 3.8243E-06 Butyric acid 28.210 1.1040E+00 -2.8523E-03 2.9528E-06 Carbon disulfidc 39.938 2.35658-01-7.20986-04 1.0443E-06 Carbon dioxide -338.956 5.2796E+00 -2.3279E-02 3.5980E-05 Carbon monoxide - 19.3122.5072E+00 -2.8970E-02 1.2745E-04 Carbon tetrachloride 9.671 9.3363E-01 -2.6768E-03 3.04250-06 Chlorine 127.601 6.0215E-01 1.5776E-03 -5.30990-07 Chloroform 28.296 6.58970-01 -2.0353E-03 2.5901E-06 Cyclohexane -44.417 1.6016E+00 -4.4676E-03 4.75828-06 Cyclohexanol -47.321 1.9131E+00 -4.8388E-03 4.7281E-06 Cyclopropane 30.543 5.0198E-01 -2.10406-03 3.7444E-06 Dichloromethane 38.941 4.90086-01-16224E-03 2.3069E-06 Diethyl ether 75.939 7.7335E-01 -2.7936E-03 4.4383E-06 Diethyl ketone 26.231 1.2822E+00 -3.7449E-03 4.3816E-06 Dimethylamine 36.962 9.5817E-01 -3.5846E-03 5.39906-06 Ethane 38.332 4.1006E 01 -2.3024E-03 5.9347E-06 Ethanol 59.342 3.6358E-01 -1.2164E-03 1.8030E-06 Ethyl acetate 62.832 8.4097E-01 -2.6998E-03 3.6631E-06 Ethyl chloride 60.180 3.4553E-01 -1.2983E-03 2.1963E-06 Ethylbenzene 102.11 5.5959E-01 -1.5609E-03 2.0149E-06 Ethylene 25.597 5.7078E-01 -3.3620E-03 8.4120E-06 Ethylene glycol 75.878 6.4182E-01 -1.6493E-03 1.6937E-06 Ethylene oxide 35.720 4.2908E-01 -1.5473E-032.4070E-06 Fluorine 83.829 -7.8518E-01 5.2305E-03 4.6617E-06 Glycerol 132.145 8.6007E-01 -1.9745E-03 1.8068E-06 Hydrogen 50.607 -6.1136E+00 3.0930E-01 -4.1480E-03 Hydrogen chloride 73.993 -1.2946E-01 -7.8980E-05 2.6409E-06 Hydrogen cyanide -123.155 1.7769E+00 -5.8083E-03 6.9129E-06 Hydrogen peroxide -15.248 6.7693E-01 -1.4948E-03 1.2018E-06 i-Butane (iso-Butane) 71.791 4.8472E-01 -2.0519E-03 4.06348-06 Methane -0.018 1.1982E+00 -9.8722E-03 3.1670E-05 Methanol 40.152 3.1046E-01-1,0291E-03 1.45988-06 T min 244 238 177 238 165 286 185 89 208 221 172 148 151 291 201 179 241 195 268 280 269 163 218 69 251 172 211 281 298 147 179 158 235 182 91 160 191 138 179 105 261 162 53 292 14 165 261 273 115 92 176 Tmx 491 542 471 505 383 528 507 378 555 536 510 483 415 533 512 457 617 385 629 506 565 497 274 120 501 396 483 498 563 358 459 420 505 394 275 465 471 414 555 254 581 422 137 651 32 308 40 694 367 172 461 130.72 74.23 112.49 162.07 218.25 92.42 101.98 175.81 191.76 147,08 107.40 170.66 106,00 183.60 165.52 89.90 98.37 73.52 85.55 141.61 79.93








