Question
Calculate the succesive equilibrium potentials for Na+, K, and Cl- at 20 degrees Celsius. -_^) 30.0 E = Na+ RX T DAY 1 10:22 PROGRESS:

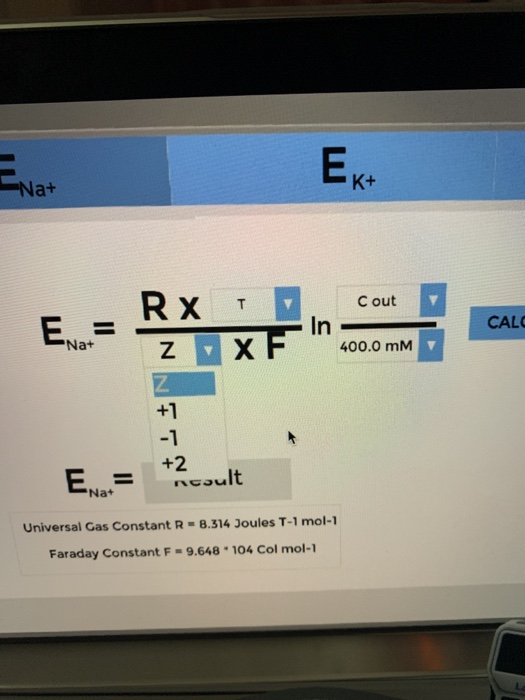
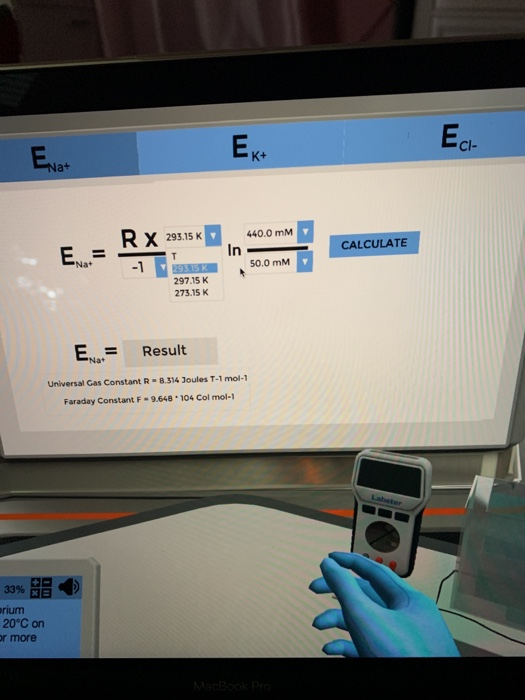
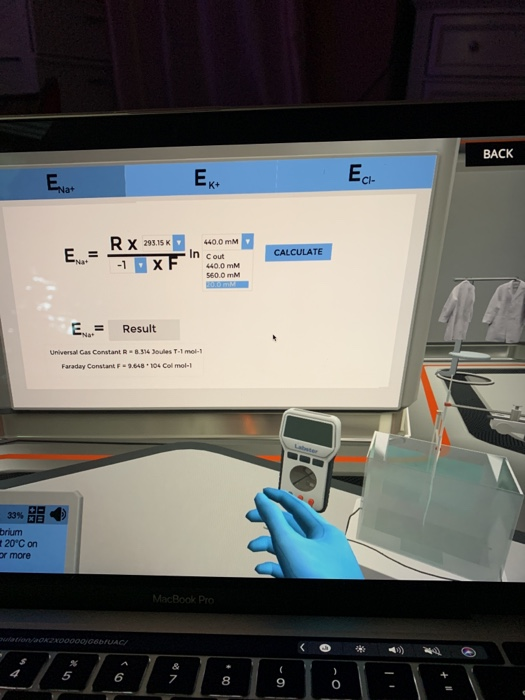


-_^) 30.0 E = Na+ RX T DAY 1 10:22 PROGRESS: 33% Calculate the successive equilibrium potentials for Na+, K+ and Ch at 20C on the screen. Raise the LabPad for more information. = Na ZXF Ek+ In Result Universal Gas Constant R-8.314 Joules T-1 mol-1 Faraday Constant F-9.648 104 Col mol-1 Cout 400.0 mm CALCULATE Ecl-
Step by Step Solution
3.60 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Nernst equation Equilibrium potentialERTZFln C out ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



