Question
Calculate the temperature at which the following reaction becomes spontaneous. Compound AH% (kJ) AS mol (K. mol) J Cl2(g) 0.0 +223.1 CINO2(g) +12.6 +272.2
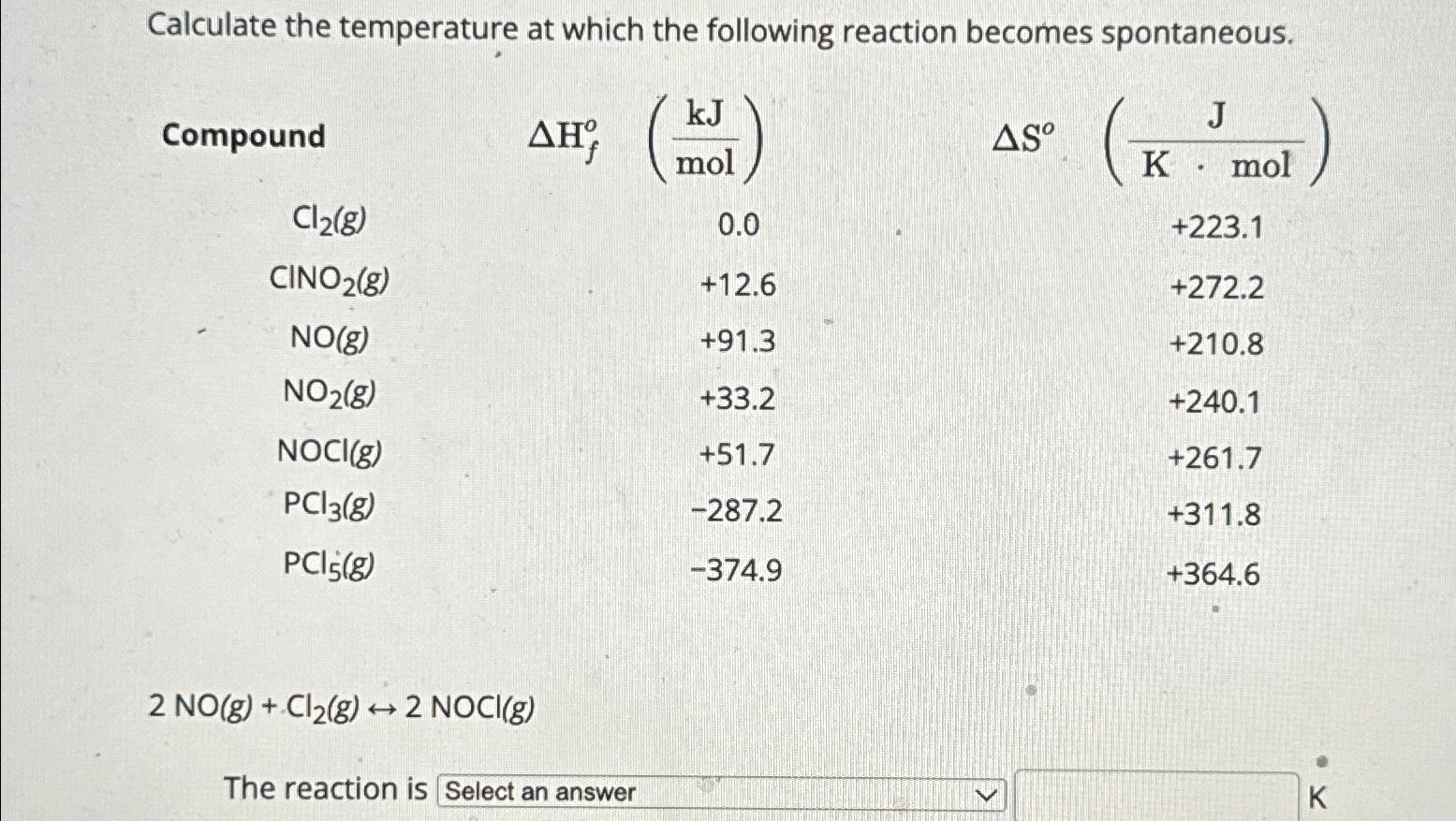
Calculate the temperature at which the following reaction becomes spontaneous. Compound AH% (kJ) AS mol (K. "mol) J Cl2(g) 0.0 +223.1 CINO2(g) +12.6 +272.2 NO(g) +91.3 +210.8 NO2(g) +33.2 +240.1 NOCI(g) +51.7 +261.7 PC13(g) -287.2 +311.8 PCl5(g) -374.9 +364.6 2 NO(g) + Cl2(g) 2 NOCI(g) The reaction is Select an answer K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Chemistry Coursebook
Authors: Lawrie Ryan, Roger Norris
2nd Edition
1316637735, 978-1316637739
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



