Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can i get help with the answer with the values Experiment 2.1 Absorption Spectrum of a Conjugated Dye Introduction is well known that comedy have
can i get help with the answer with the values 

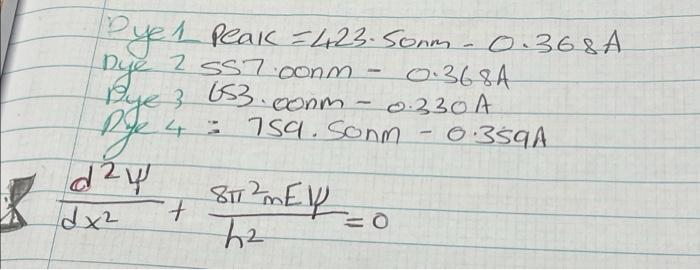
Experiment 2.1 Absorption Spectrum of a Conjugated Dye Introduction is well known that comedy have ahsario hands at logger and longer wavelengths is the band is in the visible region of the electromete spectrum. These general features of a conjugate de of conjugated double bonds in Inc, long compted systems are often coloured, le, the bond system can be understood on the basis of a very simple model for the electrons of the molecule model forms the basis for quantum theory and is known as the particle in a one-dimensional box The aim of this experiment is to determine the allowed energies and the position probability fun of a particle, that can only move in one dimension and is confined to a region of length, a the potential is infinite. This is clearly impossible and consequently the probability of finding the axis), and between x = 0 and x-a the potential energy is zero and outside this region (x > 0,X Suppose a particle of mass, m. (ie, an electron) is restricted to motion in one dimension (eg. Whe electron outside this restricted region is zero, and hence v=0 when 0 2x2a. The one-dimensional form of Schrdinger wave equation is given by: dav 8 my + =0 dx2 (1) where y is the probability of finding the electron of mass m and energy E in a box of length a. distance and h is Planck's constant. The solution of this equation, which satisfies the boundary conditions, can be written as: (2) v=A sin (nax/a) where n = 1, 2, 3 ... and A is a constant factor. The probability function corresponding to this solution function, or the Eigen function, is: - Asia) These boundary conditions Tetrict Ove allowed levels of certain discrete cycles (3) E. ? Bma The integer, Ti, is termed the quantum number. Note that the energy of the particle cannot have a continuous range of values but is quised in units of him The Pauli Exclusion principle stipulates that no two electrons can have the same quantum numbers no more than two electrons, one with the spins and one with spin can be assigned wave function, with a value of n The ground state of the molecule with N electrons will have the N2 lowest levels filled, and all higher levels empty. When the molecule absorbs light this is associated with a one-electron jump from the highest occupied (filled) level (H.O.MO - Highest Occupied Molecular Orbital), 'n to the lowest unoccupied (empty) level (LUMO - Lowest Unoccupied Molecular Orbital). 12 Consequently, to a particular In this experiment, the absorption spectrum of a series of conjugated dyes will be examined. The series of dye is: S etee 1 HLH HI Et Et 1 = 3,3' - diethylthiacyanine iodide. when j-0. 11 = 3,3'-diethylthiacarbocyanine iodide when j=1. III = 3,3' - diethylthiadicarbocyanine iodide......... when j-2. IV = 3,3' - diethylthiatricarbocyanine iodide....... when j-3. Byen = Sonn- 1 Peak - 423. Sorm - 0.368A Dye 2 ss7.com 0.3684 Bye 3 663.com - 0.330A ge 4 : 759. Sonm 0359A day 817 dx 7 h2 - ST? MEY=0 

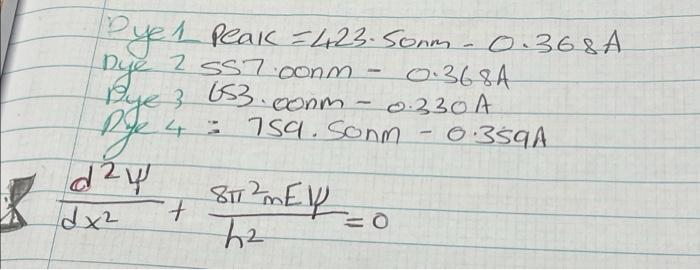
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


