Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please check my work for question #1; thank you Unkmown: F36 SolidOrganicSolventSolidOrganicSolventwithUnknownTrials#1#2Mass(g)15.985g2.052gInitialTemperature(C)89.5C89.0CTemperature(C)43.8C56.5CFinalTemperature(C)43.6C37.5C a) Solid Organic Solvent T=imKfm=iKfT=(1)(4.53mC)45.9C=10.13245033m=T=TfTi=43.6C89.5C=45.9C b) Solid Organic Solvent with Unknown
can you please check my work for question #1; thank you 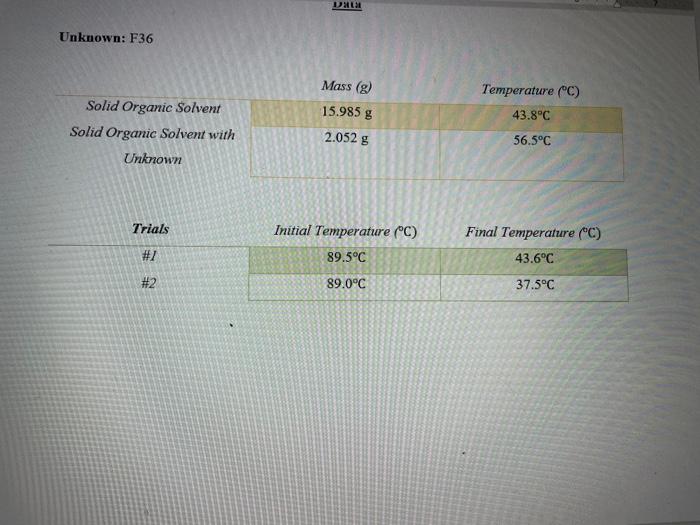
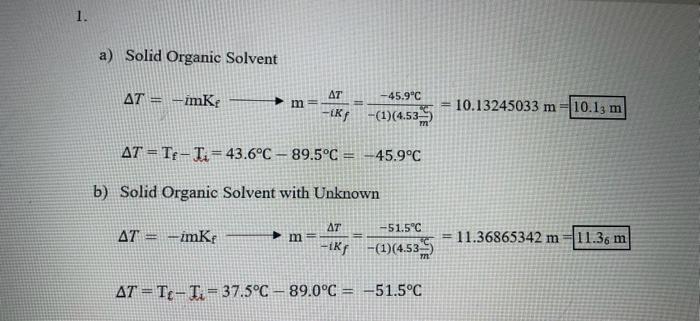


Unkmown: F36 SolidOrganicSolventSolidOrganicSolventwithUnknownTrials#1#2Mass(g)15.985g2.052gInitialTemperature(C)89.5C89.0CTemperature(C)43.8C56.5CFinalTemperature(C)43.6C37.5C a) Solid Organic Solvent T=imKfm=iKfT=(1)(4.53mC)45.9C=10.13245033m=T=TfTi=43.6C89.5C=45.9C b) Solid Organic Solvent with Unknown T=imKfm=iKfT=(1)(4.53mC)51.5C=11.36865342m=T=TTi=37.5C89.0C=51.5C You will repeat this process after dissolving your unknown in the solid solvent. Here your unknown is the solute. The equation that describes how much the freezing point is lowered is given by: T=imKF(Equation1) Here T is the change in freezing point (TFTi) on C. KF is called the freezing point lowering constant. KF=4.53C/m for your solid organic solvent. Here m is the molality of the solution and i is the van't Hoff factor. Because your unknowns are all molecular, i=1 m=kilogramsofsolventmolesofsolute(Equation2) You will measure or be given everything in Equations 1 and 2 except for the moles of the solute (your unknown). You will solve Equation 1 for the molality, m, of your solution. You will then solve Equation 2 for the moles of your unknown in the solution. The molar mass of your unknown is just the mass of your unknown divided by the moles of your unknown. Calculations: 1.) Find Tr for your experiment using the two freezing points you found in the experiment. 2.) Find the molality of your solution with your unknown dissolved in your solid organic 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


