Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you please go over 2, 3, 4, and 6? Thank you by always contain acids. E) conduct heat. 2. What is the vapor pressure

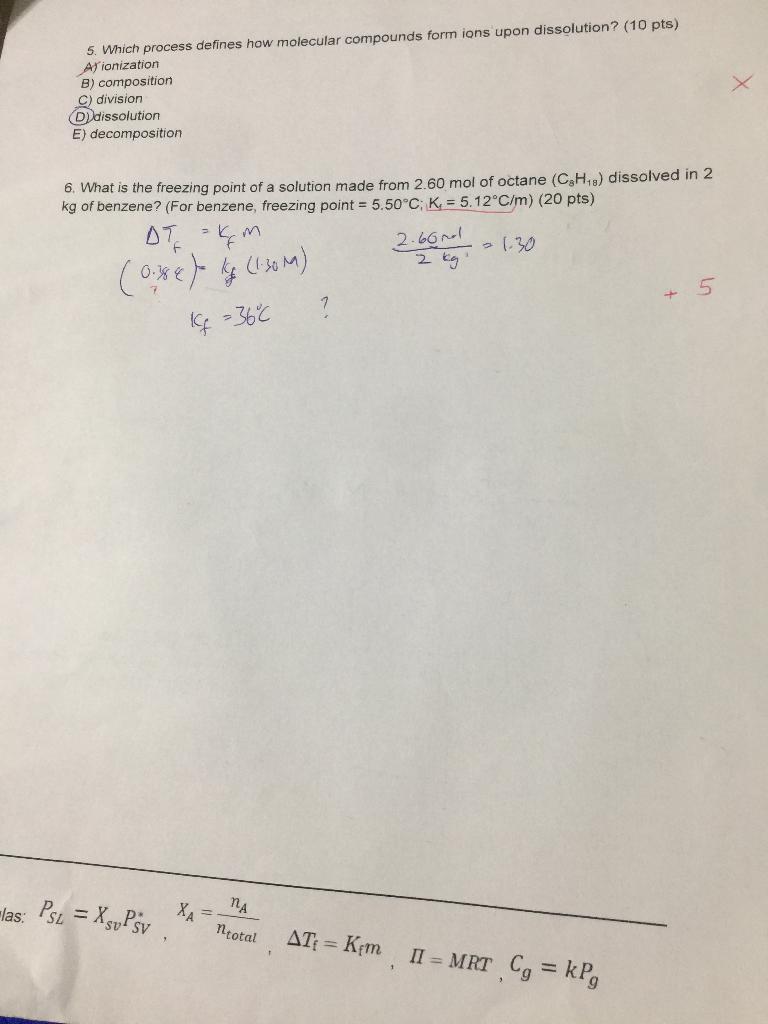
Can you please go over 2, 3, 4, and 6? Thank you
by always contain acids. E) conduct heat. 2. What is the vapor pressure above a solution prepared by dissolving 0.500mol of a nonvolatile solute in 3.19mol of hexane at 49.6C ? P hexsen =400.0 torr at 49.6C. (25 pts) Psc=XsuPsy(319+0.5)319=(3.199)(400torr)(10.500)(40tirr)=1076twe 3. If the molality of ions in an aqueous solution of MgF2 is 0.930m and the salt dissociates completely, what is the molality of MgF2 ? (20 pts) 1L0.930mMgF2=0.930MMgF2 4. If a general chemistry student adds 200.0g of sugar to a cup of water (250mL) at room-temperature, what type of solution is obtained? Show your work. (at room temperature: solubility of sugar =91.0g per 100g of water; density of water =1g/mL)(15pts) A) Unsaturated B) Saturated C) Soluted (D) Supersaturated E) Oversaturated W. Wich process defines how molecular compounds form ions upon dissolution? ( 10 pts) A) ionization B) composition C) division D) dissolution E) decomposition 6. What is the freezing point of a solution made from 2.60 mol of octane (C8H18) dissolved in 2 kg of benzene? (For benzene, freezing point =5.50C;K1=5.12C/m)(20pts) Tf(0.38)kf=kfm=kf(1.30M)2kg2.66N=1.30=36C +5 las: PSL=XSvPSV,XA=ntotalnA,Tf=Kfm,=MRT,Cg=kPgStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


