Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you please help with 1 and 2. A solution of an unknown nonvolatile NON ELECTROLYTE is prepared by adding 3.090 grams of the unknown
Can you please help with 1 and 2. 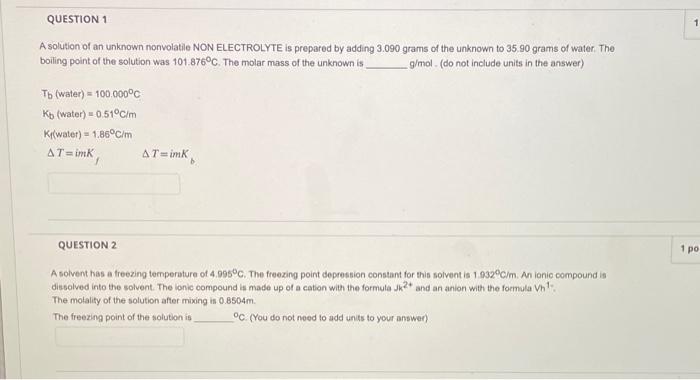
A solution of an unknown nonvolatile NON ELECTROLYTE is prepared by adding 3.090 grams of the unknown to 35.90 grams of water. The boiling point of the solution was 101.876C. The molar mass of the unknown is g/mol. (do not include units in the answer) Tb(water)=100.000CKb(water)=0.51C/mKiwater)=1.86C/mT=imKfT=imKb QUESTION 2 A solvent has a freezing temperature of 4.995C. The freezing point depression constant for this solvent is 1.032C/m. An ionic compound is dissolved into the solvent. The lonic compound is made up of a cation with the formula Jk2+ and an anion with the formula Vh1. The molality of the solution after mixing is 0.8504m. The freezing point of the solution is C. (You do not need to add units to your answen) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


