Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please show the work of the multiplication in details? Thank you The rate constant of a chemical reaction at 25C has been found
can you please show the work of the multiplication in details? Thank you
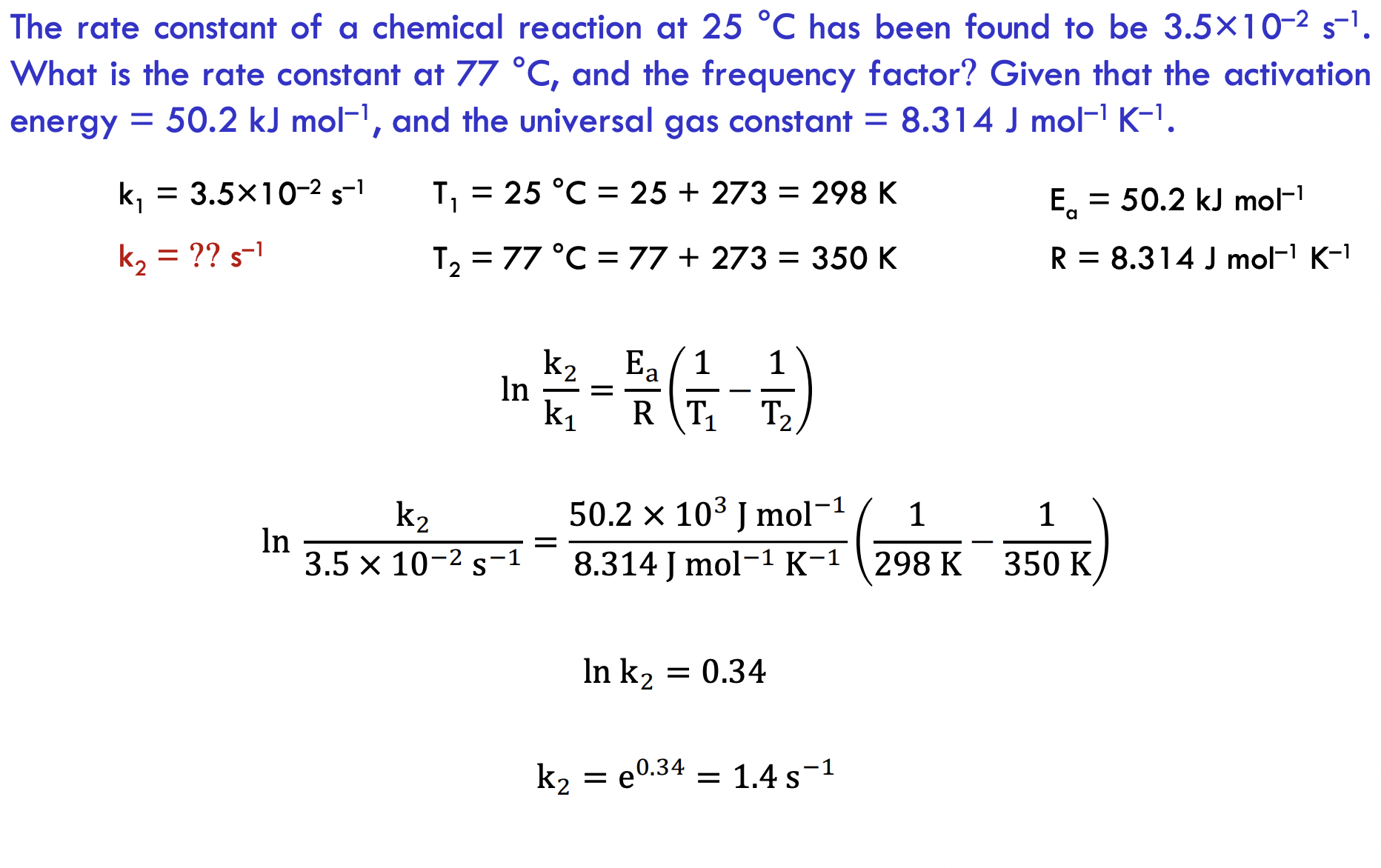 The rate constant of a chemical reaction at 25C has been found to be 3.5102s1. What is the rate constant at 77C, and the frequency factor? Given that the activation energy =50.2kJmol1, and the universal gas constant =8.314Jmol1K1. k1=3.5102s1T1=25C=25+273=298KEa=50.2kJmol1k2=??s1T2=77C=77+273=350KR=8.314Jmol1K1lnk1k2=REa(T11T21)ln3.5102s1k2=8.314Jmol1K150.2103Jmol1(298K1350K1)lnk2=0.34k2=e0.34=1.4s1
The rate constant of a chemical reaction at 25C has been found to be 3.5102s1. What is the rate constant at 77C, and the frequency factor? Given that the activation energy =50.2kJmol1, and the universal gas constant =8.314Jmol1K1. k1=3.5102s1T1=25C=25+273=298KEa=50.2kJmol1k2=??s1T2=77C=77+273=350KR=8.314Jmol1K1lnk1k2=REa(T11T21)ln3.5102s1k2=8.314Jmol1K150.2103Jmol1(298K1350K1)lnk2=0.34k2=e0.34=1.4s1 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


