Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you plz answer all the questions Thanks Ammonium compounds are very soluble in water and dissociate in an endothermic reaction. An experiment is done
Can you plz answer all the questions 
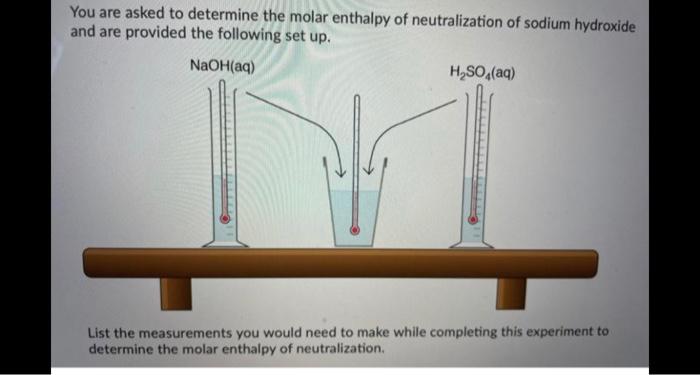

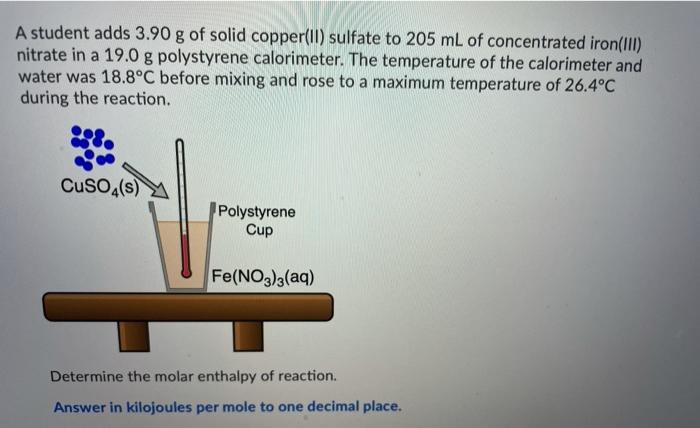
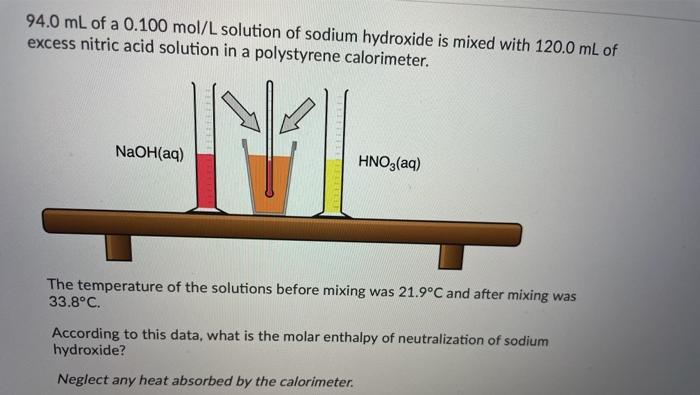
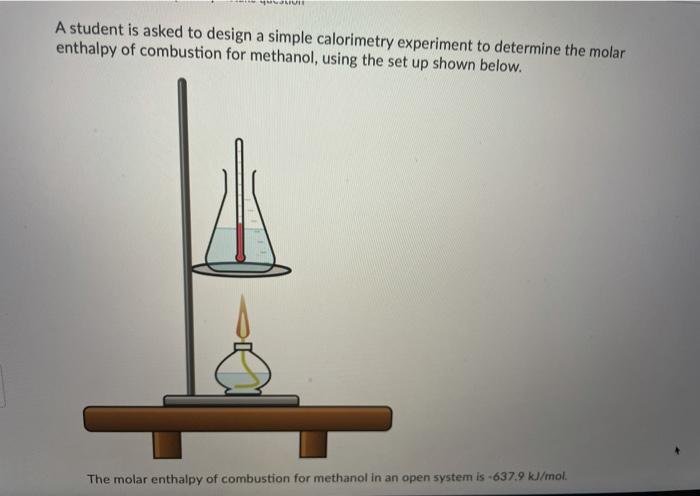
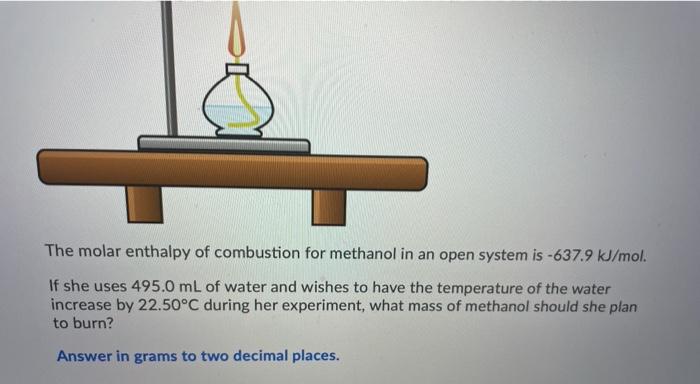
Ammonium compounds are very soluble in water and dissociate in an endothermic reaction. An experiment is done to determine which ammonium compound, ammonium chloride or ammonium nitrate, would be better to use in an instant cold pack. The general experimental design is shown below (0.50 g of the solid ammonium compound is added to water in a polystyrene cup and allowed to dissolve for three minutes) Wister NH,CHUA) on NH,NO4%) Cup In analyzing the data, it is determined that the experimentat molar enthusipy for both compounds is much lower than the theoretical molar enthalples. Using the word bank below, match each of the following: Controlled variable (Blank 11 Manipulated variable Blank 21 Responding variable (Blank 3] Improvement to reduce error Bank 41 Controlled variable (Blank 11 Manipulated variable (Blank 21 Responding variable (Blank 3] Improvement to reduce error (Blank 41 There may be more than one answer for a given blank. Please choose only one Word Banki Temperature change of water Type of ammonium compound Amount of water in calorimeter Use less water in the calorimeter Enthalpy change of ammonium compound Amount of ammonium compound dissolved Temperature change of ammonium compound Add a second Styrofoam cup to the calorimeter Dissolve a greater mass of the ammonium compound Use a digital thermometer to measure water temperature Blank #1 Blank # 2 Blank #3 Blank #4 You are asked to determine the molar enthalpy of neutralization of sodium hydroxide and are provided the following set up. NaOH(aq) H,SO (aq) List the measurements you would need to make while completing this experiment to determine the molar enthalpy of neutralization A hand-warmer packet contains a mixture of powdered iron, carbon, sodium chloride, sawdust, and zeolite, all moistened by a little water. The packet is activated by removing the plastic cover, which exposes the materials in the packet to air. The reaction that occurs is represented by the following equation. 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) AH = -1645 kJ What mass of iron must react to produce 672 kJ of energy? Answer in grams to two decimal places. A student adds 3.90 g of solid copper(ll) sulfate to 205 mL of concentrated iron(III) nitrate in a 19.0 g polystyrene calorimeter. The temperature of the calorimeter and water was 18.8C before mixing and rose to a maximum temperature of 26.4C during the reaction. CuSO4(s) Polystyrene Cup Fe(NO3)3(aq) Determine the molar enthalpy of reaction. Answer in kilojoules per mole to one decimal place. 94.0 mL of a 0.100 mol/L solution of sodium hydroxide is mixed with 120.0 mL of excess nitric acid solution in a polystyrene calorimeter. NaOH(aq) INI HNO3(aq) The temperature of the solutions before mixing was 21.9C and after mixing was 33.8C. According to this data, what is the molar enthalpy of neutralization of sodium hydroxide? Neglect any heat absorbed by the calorimeter. ORII A student is asked to design a simple calorimetry experiment to determine the molar enthalpy of combustion for methanol, using the set up shown below. The molar enthalpy of combustion for methanol in an open system is -637.9 kl/mol. The molar enthalpy of combustion for methanol in an open system is -637.9 kJ/mol. If she uses 495.0 mL of water and wishes to have the temperature of the water increase by 22.50C during her experiment, what mass of methanol should she plan to burn? Answer in grams to two decimal places Thanks







Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


