Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you plz try to answer all the questions Thanks The follow graph was prepared by monitoring the temperature of 5.0 g pieces of two
Can you plz try to answer all the questions 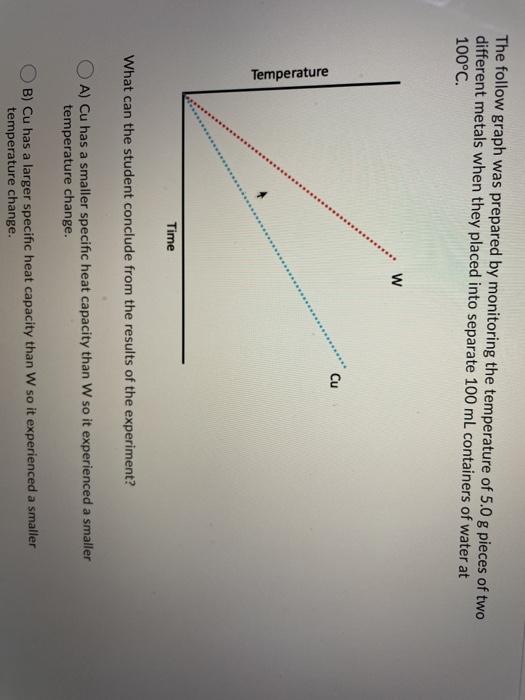

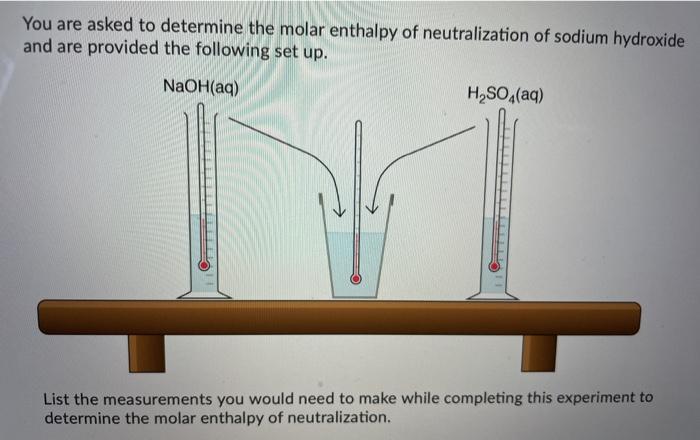
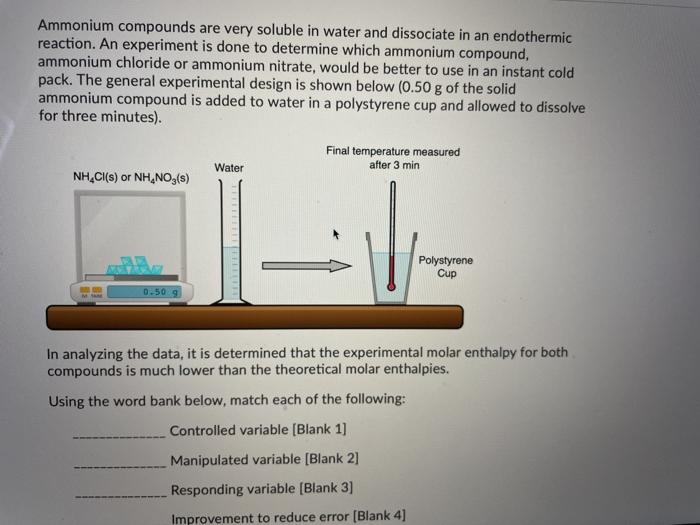
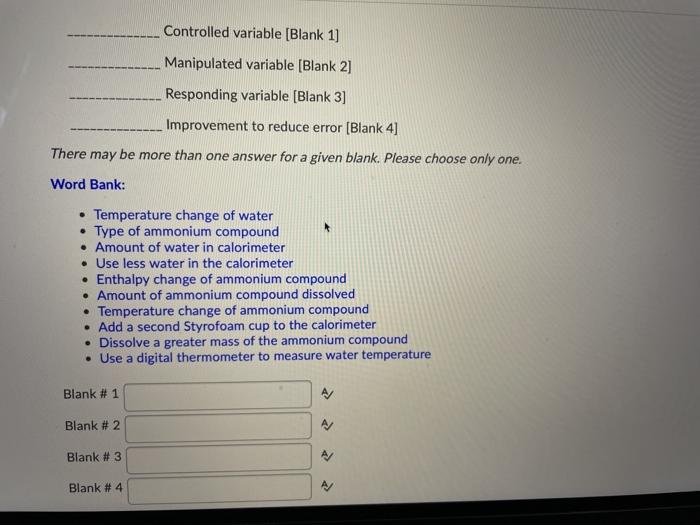
The follow graph was prepared by monitoring the temperature of 5.0 g pieces of two different metals when they placed into separate 100 mL containers of water at 100C. W Cu Temperature Time What can the student conclude from the results of the experiment? A) Cu has a smaller specific heat capacity than W so it experienced a smaller temperature change. B) Cu has a larger specific heat capacity than W so it experienced a smaller temperature change. Time What can the student conclude from the results of the experiment? A) Cu has a smaller specific heat capacity than W so it experienced a smaller temperature change. B) Cu has a larger specific heat capacity than W so it experienced a smaller temperature change. C) Cu has a smaller specific heat capacity than W so it experienced a larger temperature change. D) Cu has a larger specific heat capacity than W so it experienced a larger temperature change. You are asked to determine the molar enthalpy of neutralization of sodium hydroxide and are provided the following set up. NaOH(aq) H.SO.(aq) List the measurements you would need to make while completing this experiment to determine the molar enthalpy of neutralization. Ammonium compounds are very soluble in water and dissociate in an endothermic reaction. An experiment is done to determine which ammonium compound, ammonium chloride or ammonium nitrate, would be better to use in an instant cold pack. The general experimental design is shown below (0.50 g of the solid ammonium compound is added to water in a polystyrene cup and allowed to dissolve for three minutes) Final temperature measured Water after 3 min NH,Cl(s) or NH.NO3(s) Polystyrene Cup 0.50 9 In analyzing the data, it is determined that the experimental molar enthalpy for both compounds is much lower than the theoretical molar enthalpies. Using the word bank below, match each of the following: Controlled variable (Blank 1] Manipulated variable (Blank 2] Responding variable (Blank 3] Improvement to reduce error (Blank 4] Controlled variable (Blank 1] Manipulated variable (Blank 2] Responding variable (Blank 3] Improvement to reduce error (Blank 4] There may be more than one answer for a given blank. Please choose only one. Word Bank: Temperature change of water Type of ammonium compound Amount of water in calorimeter Use less water in the calorimeter Enthalpy change of ammonium compound Amount of ammonium compound dissolved Temperature change of ammonium compound Add a second Styrofoam cup to the calorimeter Dissolve a greater mass of the ammonium compound Use a digital thermometer to measure water temperature Blank # 1 Blank # 2 N Blank # 3 Blank #4 Thanks





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


