Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you solve table III:preparing and analyzing the equilibrium mixture Table 1: Standard solution for FNCS2+ ion Beer's plot I Solution SI (blank) S2 S3
can you solve table III:preparing and analyzing the equilibrium mixture 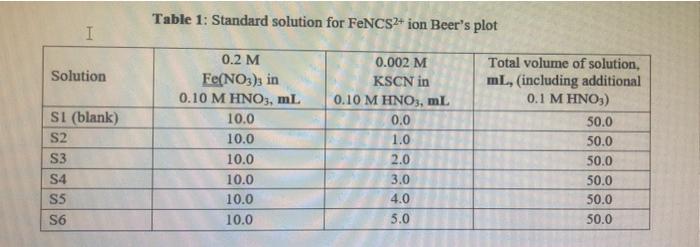
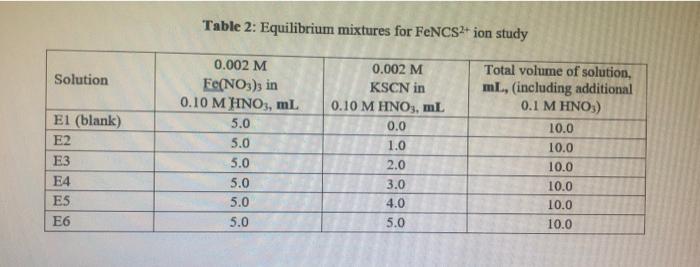
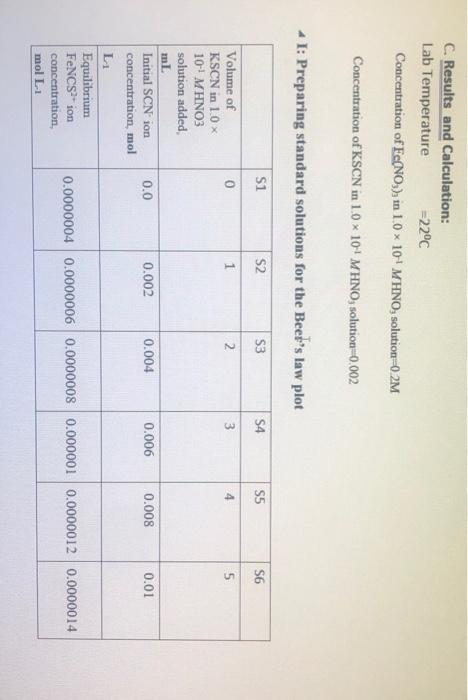
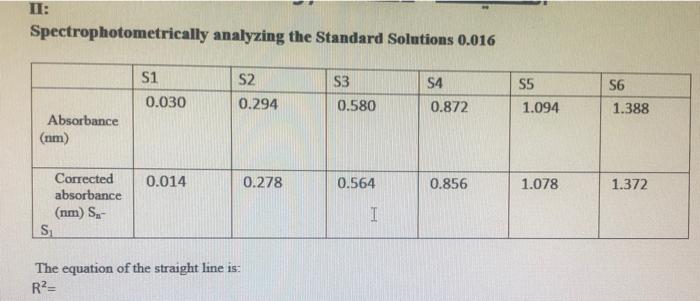
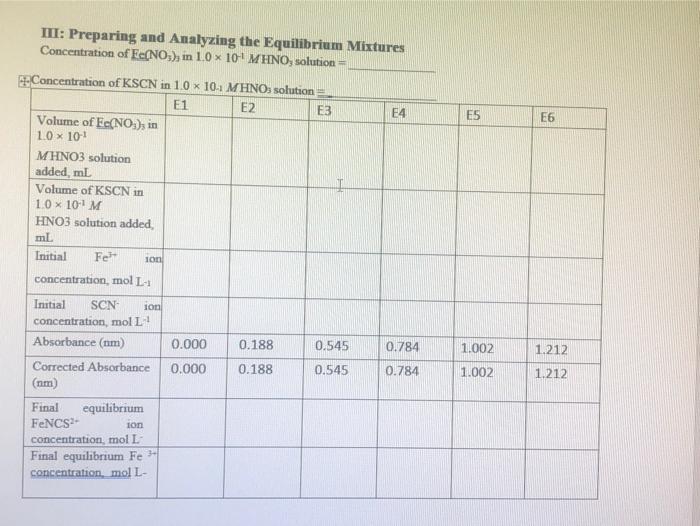
Table 1: Standard solution for FNCS2+ ion Beer's plot I Solution SI (blank) S2 S3 S4 S5 S6 0.2 M Fe(NO3)3 in 0.10 M HNO3, ml 10.0 10.0 10.0 10.0 10.0 10.0 0.002 M KSCN in 0.10 M HNO3, ml 0.0 1.0 2.0 3.0 4.0 5.0 Total volume of solution, ml, (including additional 0.1 M HNO3) 50.0 50.0 50.0 50.0 50.0 50.0 Table 2: Equilibrium mixtures for FeNCS2+ ion study Solution El (blank) E2 E3 E4 ES E6 0.002 M Fe(NO3), in 0.10 M HNO3, ml 5.0 5.0 5.0 5.0 5.0 5.0 0.002 M KSCN in 0.10 M HNO3, ml 0.0 1.0 2.0 Total volume of solution, ml, (including additional 0.1 M HNO3) 10.0 10.0 10.0 10.0 10.0 10.0 3.0 4.0 5.0 C. Results and Calculation: Lab Temperature =22C Concentration of Fe(NO), in 10 x 10 MHNO, solution=0.2M Concentration of KSCN in 1.0 x 10! MHNO3 solution=0.002 * 1: Preparing standard solutions for the Beer's law plot S1 S2 S3 S4 S5 S6 0 1 N 3 4 un 5 0.0 0.002 0.004 0.006 0.008 0.01 Volume of KSCN in 1.0 10-1 MHNO3 solution added mL Initial SCN ion concentration, mol L Equilibrium FeNCS-ion concentration mol L1 0.0000004 0.0000006 0.0000008 0.000001 0.0000012 0.0000014 II: Spectrophotometrically analyzing the Standard Solutions 0.016 S3 S1 0.030 S2 0.294 S4 0.872 SS 1.094 S6 1.388 0.580 Absorbance (nm) 0.014 0.278 0.564 0.856 1.078 1.372 Corrected absorbance (nm) S- S I The equation of the straight line is: R?= III: Preparing and Analyzing the Equilibrium Mixtures Concentration of Fe(NO3), in 10 x 10' MHNO, solution = E4 E5 E6 Concentration of KSCN in 10 x 10.. MHNOs solution = E1 E2 E3 Volume of Fe(NO3), in 1.0 10 MHNO3 solution added, ml Volume of KSCN in 1.0 x 10-1 M HNO3 solution added ml Initial Fe son concentration, mol L-1 0.000 0.188 0.545 0.784 1.002 1.212 0.000 0.188 0.545 0.784 1.002 1.212 Initial SCN ion concentration, moll Absorbance (nm) Corrected Absorbance (nm) Final equilibrium FeNCS- con concentration, moll Final equilibrium Fe - concentration, mol L 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


