Answered step by step
Verified Expert Solution
Question
1 Approved Answer
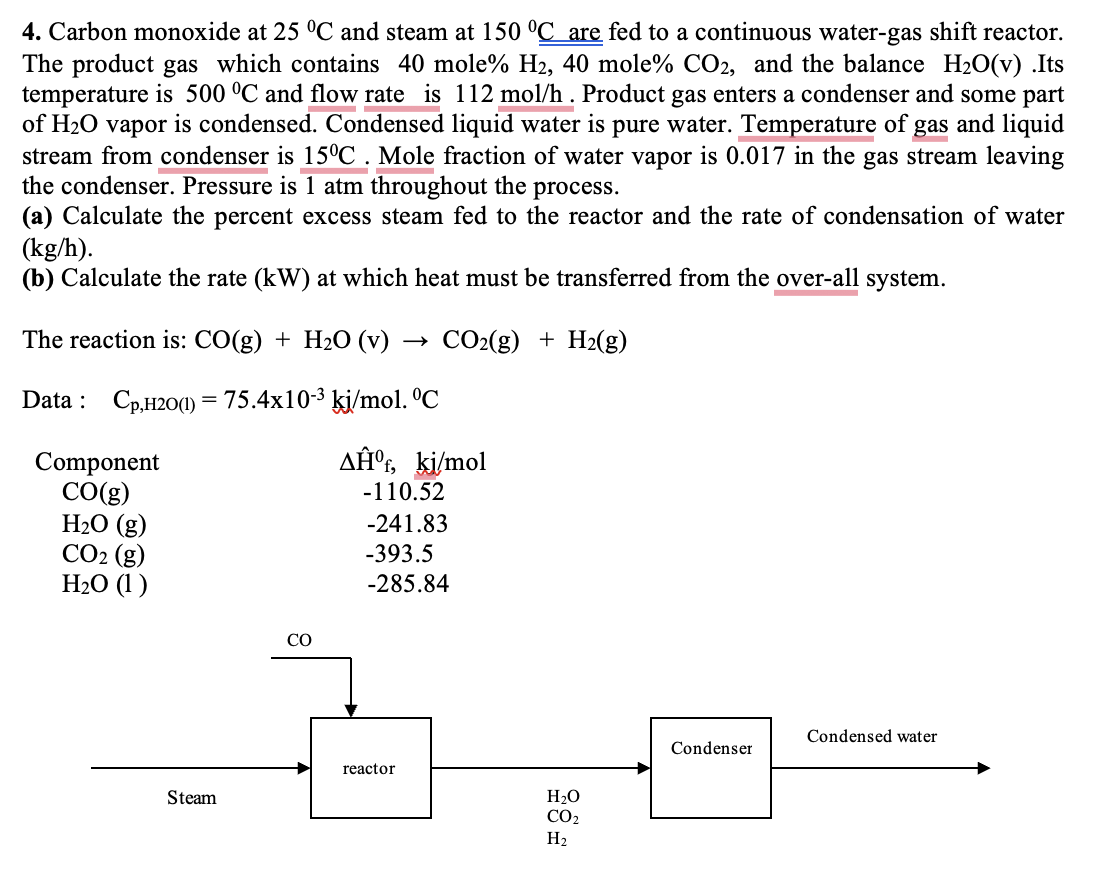
Carbon monoxide at 2 5 C and steam at 1 5 0 C ? are fed to a continuous water - gas shift reactor. The
Carbon monoxide at and steam at are fed to a continuous watergas shift reactor.
The product gas which contains molemole and the balance Its
temperature is and flow rate is Product gas enters a condenser and some part
of vapor is condensed. Condensed liquid water is pure water. Temperature of gas and liquid
stream from condenser is Mole fraction of water vapor is in the gas stream leaving
the condenser. Pressure is atm throughout the process.
a Calculate the percent excess steam fed to the reactor and the rate of condensation of water
b Calculate the rate at which heat must be transferred from the overall system.
The reaction is:
Data :
Co
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


