Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Carbon monoxide (CO) and nitrogen (N2) initially reside in the apparatus and at the states shown below. CO Tco,1-27 C Pco, 1 bar =>
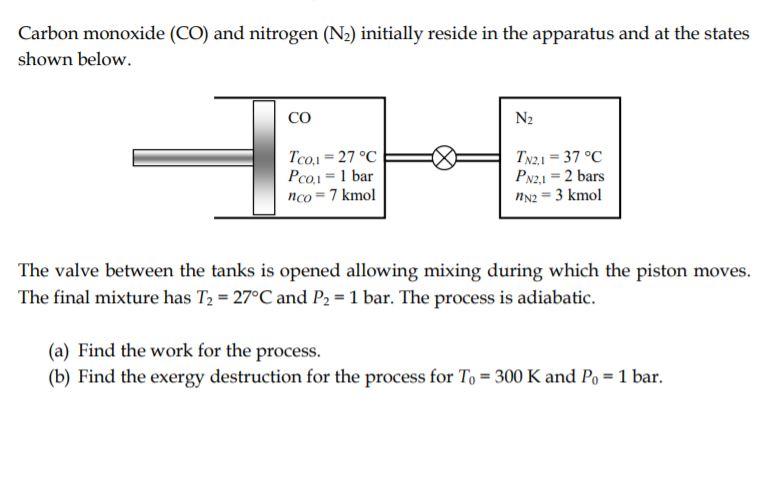
Carbon monoxide (CO) and nitrogen (N2) initially reside in the apparatus and at the states shown below. CO Tco,1-27 C Pco, 1 bar => = nco = 7 kmol N2 TN2.1-37 C PN2,1-2 bars nN2 = 3 kmol The valve between the tanks is opened allowing mixing during which the piston moves. The final mixture has T2 = 27C and P2 = 1 bar. The process is adiabatic. (a) Find the work for the process. (b) Find the exergy destruction for the process for To = 300 K and Po = 1 bar.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


