Question
Carbonic Acid has two pKas: 6.37 and 10.32. The dissociation of H2CO3 can be written as: Suppose a pure solution of H2CO3 was titrated with
Carbonic Acid has two pKas: 6.37 and 10.32. The dissociation of H2CO3 can be written as:
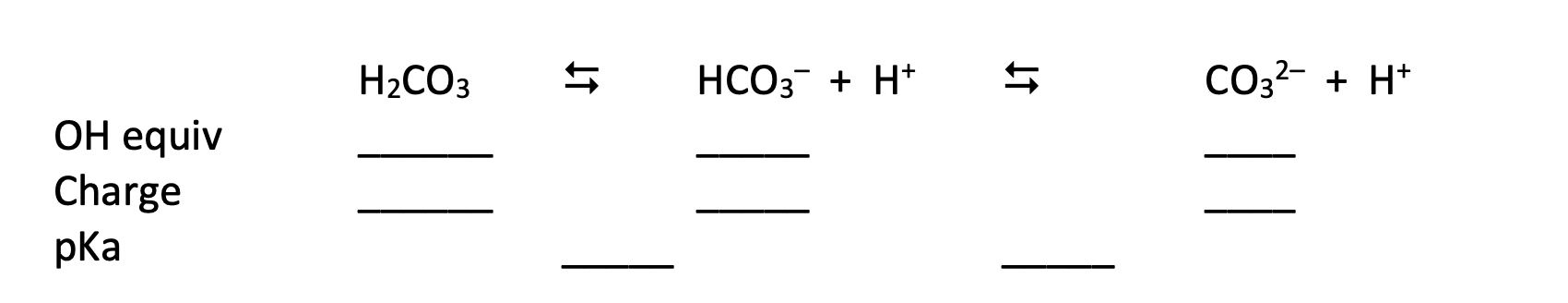
Suppose a pure solution of H2CO3 was titrated with NaOH.
a) In the spaces provided indicate at how many OH equivalents would we need to add to have about 100% of carbonic acid (H2CO3), bicarbonate ions (HCO3) or carbonate ions (CO32). Indicate also the net charge of each of these species and the pKa that is related to the first and second equilibria.
b) At how many OH equivalents do we have the point where [H2CO3] = [HCO3]? What is the pH of the solution at this point?
c) At how many OH equivalents do we have the point where [HCO3] = [CO32]? What is the pH of the solution at this point? What is the average charge of all molecules at this point?
d) Which species (H2CO3 or HCO3 or CO32) predominate when the pH is 11?
e) Which species does not exist at concentration at any measurable concentration at pH 5.5?
Please answer all parts and show all work- Thank you!
OH equiv H2CO3HCO3+H+CO32+H+ Charge pKaStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


