Answered step by step
Verified Expert Solution
Question
1 Approved Answer
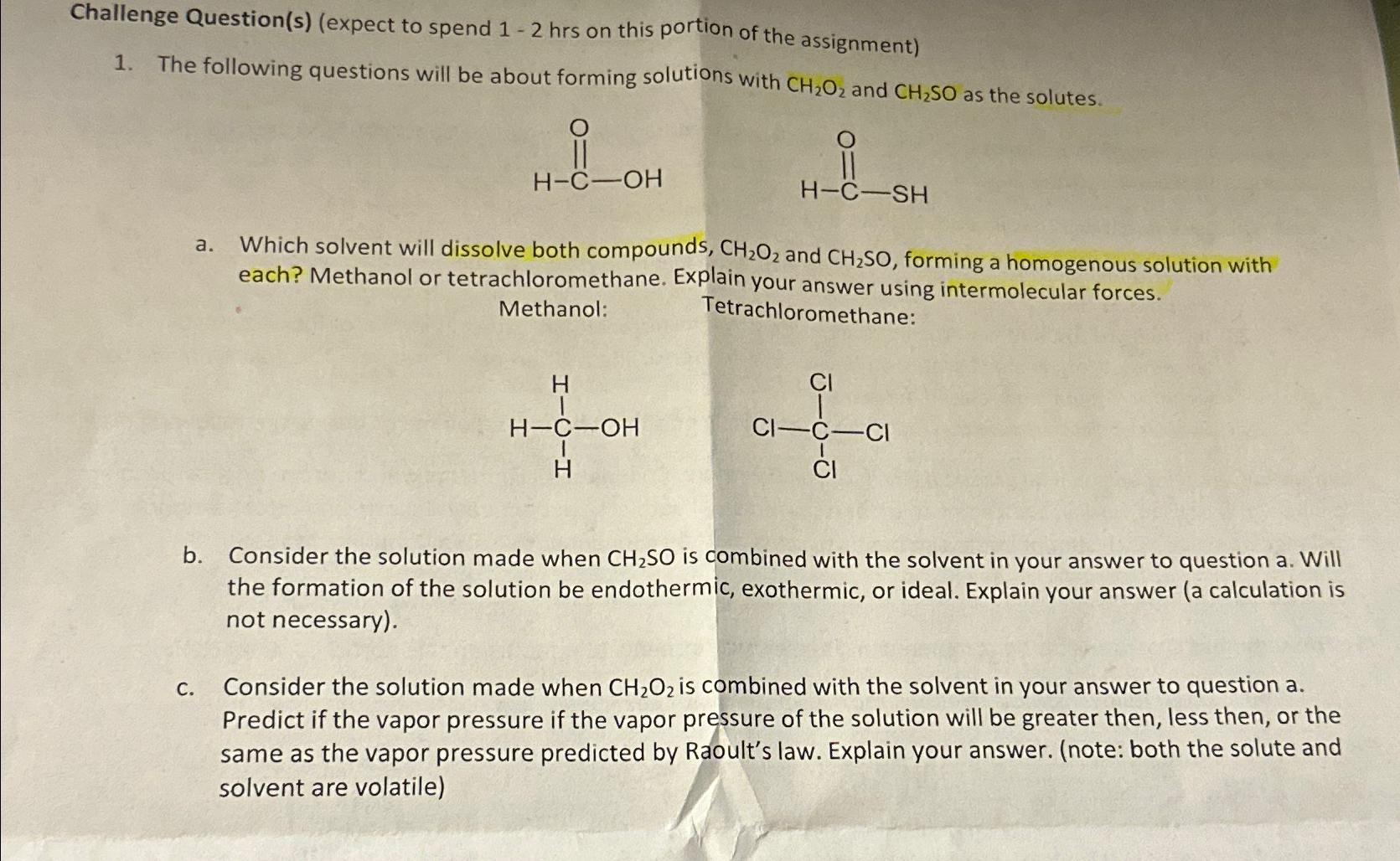
Challenge Question ( s ) ( expect to spend 1 - 2 hrs on this portion of the assignment ) The following questions will be
Challenge Questionsexpect to spend hrs on this portion of the assignment
The following questions will be about forming solutions with and as the solutes.
a Which solvent will dissolve both compounds, and forming a homogenous solution with each? Methanol or tetrachloromethane. Explain your answer using intermolecular forces.
Methanol:
Tetrachloromethane:
b Consider the solution made when is combined with the solvent in your answer to question a Will the formation of the solution be endothermic, exothermic, or ideal. Explain your answer a calculation is not necessary
c Consider the solution made when is combined with the solvent in your answer to question a Predict if the vapor pressure if the vapor pressure of the solution will be greater then, less then, or the same as the vapor pressure predicted by Raoult's law. Explain your answer. note: both the solute and solvent are volatile
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


