Question: Characteristics of Covalent and Ionic Compounds Consider table 1 at the right in answering the following questions: 1. Are the elements in the covalent
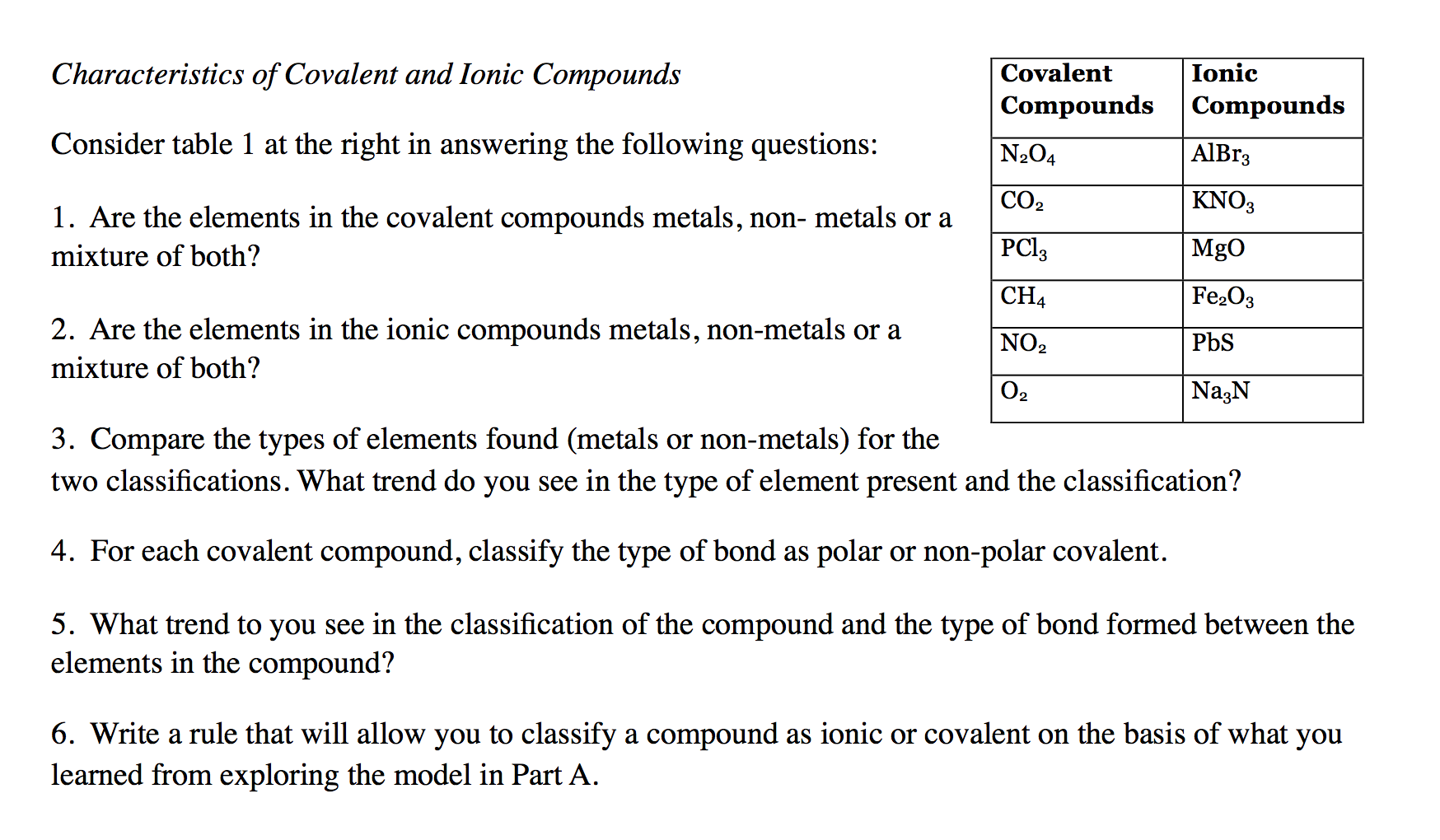
Characteristics of Covalent and Ionic Compounds Consider table 1 at the right in answering the following questions: 1. Are the elements in the covalent compounds metals, non- metals or a mixture of both? Covalent Ionic Compounds Compounds N2O4 AlBr3 CO2 KNO3 PC13 MgO CH4 Fe2O3 2. Are the elements in the ionic compounds metals, non-metals or a mixture of both? NO2 PbS 02 Na3N 3. Compare the types of elements found (metals or non-metals) for the two classifications. What trend do you see in the type of element present and the classification? 4. For each covalent compound, classify the type of bond as polar or non-polar covalent. 5. What trend to you see in the classification of the compound and the type of bond formed between the elements in the compound? 6. Write a rule that will allow you to classify a compound as ionic or covalent on the basis of what you learned from exploring the model in Part A.
Step by Step Solution
There are 3 Steps involved in it
Step1 The elements in the covalent compounds column are nonmetals Step 2 solution 1 Covalent compoun... View full answer

Get step-by-step solutions from verified subject matter experts


