Answered step by step
Verified Expert Solution
Question
1 Approved Answer
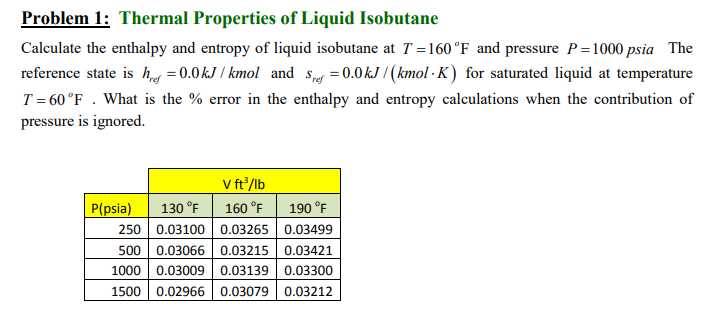
Che 301 class. Please help me. I will rate it. Thank you. Problem 1: Thermal Properties of Liquid Isobutane Calculate the enthalpy and entropy of
Che 301 class. Please help me. I will rate it. Thank you.
Problem 1: Thermal Properties of Liquid Isobutane Calculate the enthalpy and entropy of liquid isobutane at T =160 F and pressure P=1000 psia The reference state is hier = 0.0 kJ / kmol and Spep = 0.0 kJ/(kmolK) for saturated liquid at temperature T =60F . What is the % error in the enthalpy and entropy calculations when the contribution of pressure is ignored. Vft/lb P(psia) 130 F 160 F 190 F 250 0.03100 0.03265 0.03499 500 0.03066 0.03215 0.03421 1000 0.03009 0.03139 0.03300 1500 0.02966 0.03079 0.03212 Problem 1: Thermal Properties of Liquid Isobutane Calculate the enthalpy and entropy of liquid isobutane at T =160 F and pressure P=1000 psia The reference state is hier = 0.0 kJ / kmol and Spep = 0.0 kJ/(kmolK) for saturated liquid at temperature T =60F . What is the % error in the enthalpy and entropy calculations when the contribution of pressure is ignored. Vft/lb P(psia) 130 F 160 F 190 F 250 0.03100 0.03265 0.03499 500 0.03066 0.03215 0.03421 1000 0.03009 0.03139 0.03300 1500 0.02966 0.03079 0.03212Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


