Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Che 321. Thank you. 1) ( 50 points) An existing stirred-tank reactor is to be used for a 2 nd-order liquid phase reaction. A tracer
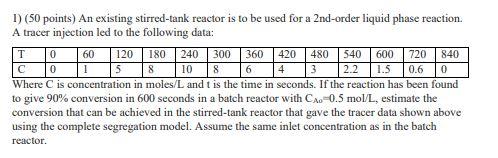
Che 321. Thank you.
1) ( 50 points) An existing stirred-tank reactor is to be used for a 2 nd-order liquid phase reaction. A tracer injection led to the following data: 5 Where C is concentration in moles/ L and t is the time in seconds. If the reaction has been found to give 90% conversion in 600 seconds in a batch reactor with CA0=0.5mol/L, estimate the conversion that can be achieved in the stirred-tank reactor that gave the tracer data shown above using the complete segregation model. Assume the same inlet concentration as in the batch reactorStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


