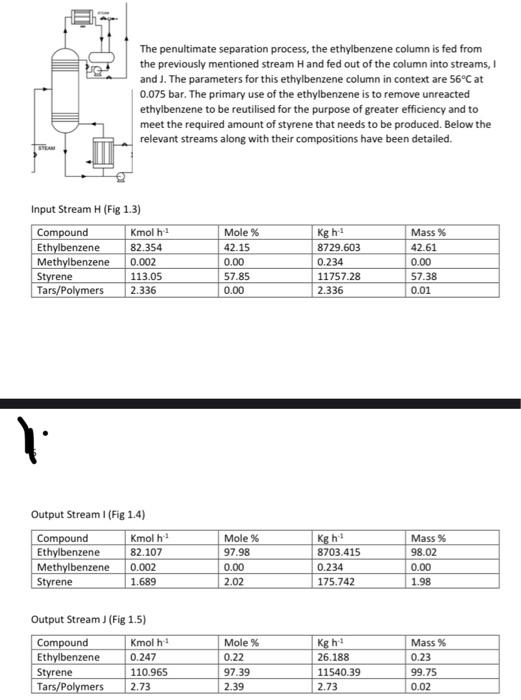
Question
Chemical engineering PLEASE ANSWER ASAP FOR GUARANTEED LIKE AND REWARD Design a distillation column for styrene Production (tars are negligible in calculations) -calculate dew point
Chemical engineering
PLEASE ANSWER ASAP FOR GUARANTEED LIKE AND REWARD
Design a distillation column for styrene Production
(tars are negligible in calculations)
-calculate dew point & bubble points
-calculate wt% leaving at top and bottom of the column
-calculate the relative volatility of top and bottom values, as well as an average value of each
-calculate number of actual trays ( minimum amount and actual amount)
-calculate reflux ratio(underwood)
-calculate a reflux ration and number of trays that work efficiently
-calculate total height to Column
-calculate the column diameter
-calculate the number of plates at rectifying and stripping section
-calculate plate diameter (after deciding which plate is most efficient
-calculate cost approximation of the column and trays ( give separate amounts and a combined total equipment cost)
IF ANYTHING ELSE REQUIRED THEN PLEASE ASK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


