Question
CHEMICAL ENGINEERING Please provide a detailed solution given that I am a beginner in the subject. Thanks An air conditioner, basically made up of a
CHEMICAL ENGINEERING
Please provide a detailed solution given that I am a beginner in the subject. Thanks
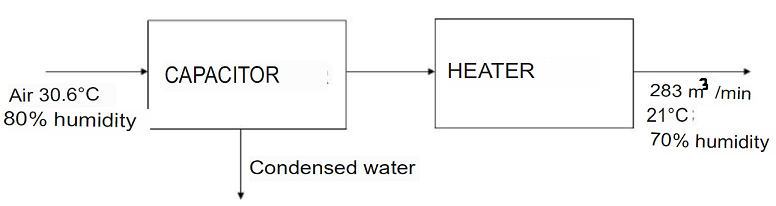
An air conditioner, basically made up of a condenser and a heater (see figure), must supply 283 m3/min at a temperature and relative humidity of 21C and 70% respectively. If the air conditioner is running on a summer day, where the air temperature and relative humidity are 30.6C and 80% respectively, calculate:
a) Mass flow rate of condensing water (SOL: 3.8 kg/min).
b) Temperature of air leaving the capacitor (SOL: 15 C).
c) Net energy contributed or eliminated in the conditioner (SOL: -218kW).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


