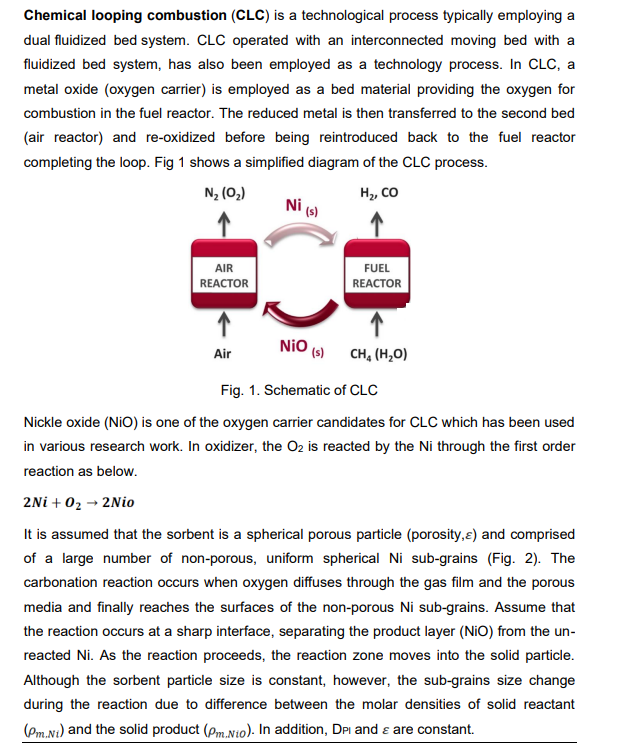
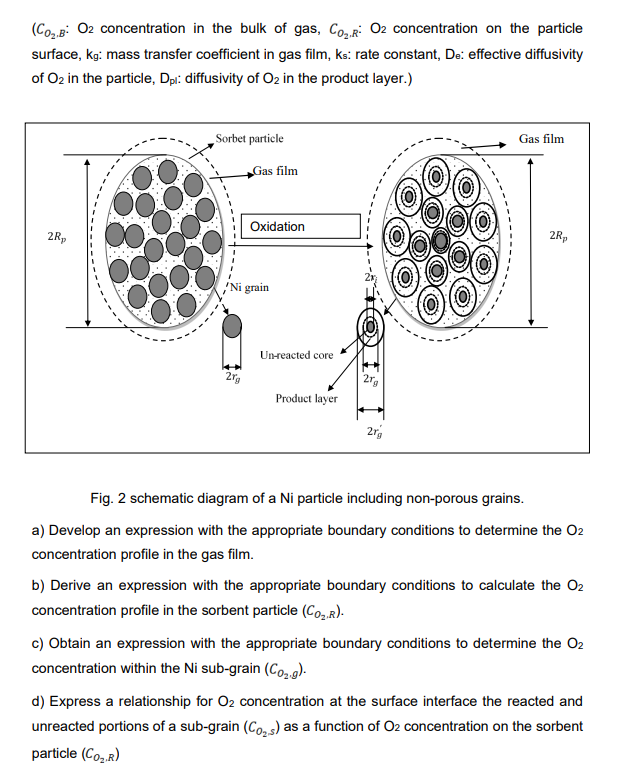
Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide (oxygen carrier) is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed (air reactor) and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig. 1. Schematic of CLC Nickle oxide (NiO) is one of the oxygen carrier candidates for CLC which has been used in various research work. In oxidizer, the O2 is reacted by the Ni through the first order reaction as below. 2Ni+O22Nio It is assumed that the sorbent is a spherical porous particle ( porosity, ) and comprised of a large number of non-porous, uniform spherical Ni sub-grains (Fig. 2). The carbonation reaction occurs when oxygen diffuses through the gas film and the porous media and finally reaches the surfaces of the non-porous Ni sub-grains. Assume that the reaction occurs at a sharp interface, separating the product layer (NiO) from the unreacted Ni. As the reaction proceeds, the reaction zone moves into the solid particle. Although the sorbent particle size is constant, however, the sub-grains size change during the reaction due to difference between the molar densities of solid reactant (m,Ni) and the solid product (m,Nio). In addition, Dpl and are constant. (CO2,B:O2 concentration in the bulk of gas, CO2,R:O2 concentration on the particle surface, kgg : mass transfer coefficient in gas film, ks : rate constant, De : effective diffusivity of O2 in the particle, Dpl: : diffusivity of O2 in the product layer.) Fig. 2 schematic diagram of a Ni particle including non-porous grains. a) Develop an expression with the appropriate boundary conditions to determine the O2 concentration profile in the gas film. b) Derive an expression with the appropriate boundary conditions to calculate the O2 concentration profile in the sorbent particle (CO2,R). c) Obtain an expression with the appropriate boundary conditions to determine the O2 concentration within the Ni sub-grain (CO2,g). d) Express a relationship for O2 concentration at the surface interface the reacted and unreacted portions of a sub-grain (CO2,) as a function of O2 concentration on the sorbent particle(CO2,R) Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide (oxygen carrier) is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed (air reactor) and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig. 1. Schematic of CLC Nickle oxide (NiO) is one of the oxygen carrier candidates for CLC which has been used in various research work. In oxidizer, the O2 is reacted by the Ni through the first order reaction as below. 2Ni+O22Nio It is assumed that the sorbent is a spherical porous particle ( porosity, ) and comprised of a large number of non-porous, uniform spherical Ni sub-grains (Fig. 2). The carbonation reaction occurs when oxygen diffuses through the gas film and the porous media and finally reaches the surfaces of the non-porous Ni sub-grains. Assume that the reaction occurs at a sharp interface, separating the product layer (NiO) from the unreacted Ni. As the reaction proceeds, the reaction zone moves into the solid particle. Although the sorbent particle size is constant, however, the sub-grains size change during the reaction due to difference between the molar densities of solid reactant (m,Ni) and the solid product (m,Nio). In addition, Dpl and are constant. (CO2,B:O2 concentration in the bulk of gas, CO2,R:O2 concentration on the particle surface, kgg : mass transfer coefficient in gas film, ks : rate constant, De : effective diffusivity of O2 in the particle, Dpl: : diffusivity of O2 in the product layer.) Fig. 2 schematic diagram of a Ni particle including non-porous grains. a) Develop an expression with the appropriate boundary conditions to determine the O2 concentration profile in the gas film. b) Derive an expression with the appropriate boundary conditions to calculate the O2 concentration profile in the sorbent particle (CO2,R). c) Obtain an expression with the appropriate boundary conditions to determine the O2 concentration within the Ni sub-grain (CO2,g). d) Express a relationship for O2 concentration at the surface interface the reacted and unreacted portions of a sub-grain (CO2,) as a function of O2 concentration on the sorbent particle(CO2,R)








