Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical Reaction Engineering question. Please help me solve these questions with complete handwritting solutions. Please try not to take answers from other tutors. Thank you.
Chemical Reaction Engineering question. Please help me solve these questions with complete handwritting solutions. Please try not to take answers from other tutors. Thank you. Have a nice day :)
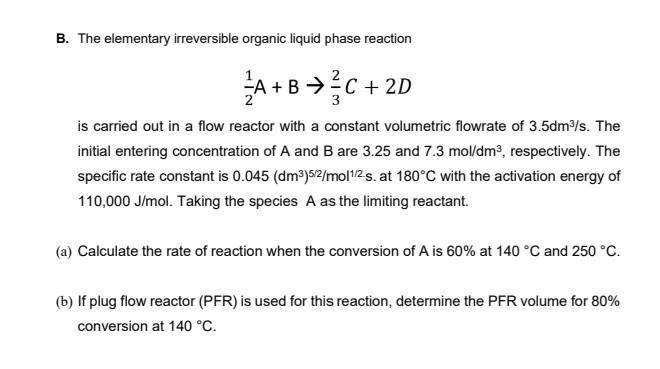
B. The elementary irreversible organic liquid phase reaction FA+B+C + 2D C is carried out in a flow reactor with a constant volumetric flowrate of 3.5dm3/s. The initial entering concentration of A and B are 3.25 and 7.3 mol/dm3, respectively. The specific rate constant is 0.045 (dm3)52/molt/2.s. at 180C with the activation energy of 110,000 J/mol. Taking the species A as the limiting reactant. (a) Calculate the rate of reaction when the conversion of A is 60% at 140 C and 250 C. (b) If plug flow reactor (PFR) is used for this reaction, determine the PFR volume for 80% conversion at 140 CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


