Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. a) What do you understand by homolytic and heterolytic bond cleavage? Show the reaction mechanism of monobromination of ethane. b) Alkanes show regular
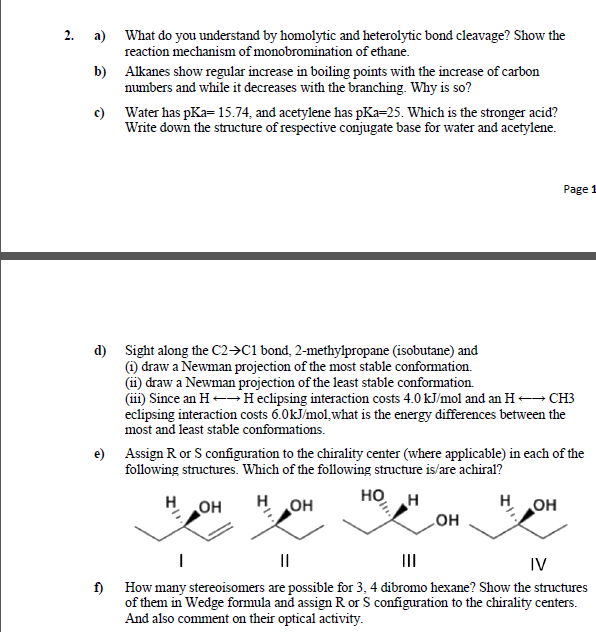
2. a) What do you understand by homolytic and heterolytic bond cleavage? Show the reaction mechanism of monobromination of ethane. b) Alkanes show regular increase in boiling points with the increase of carbon numbers and while it decreases with the branching. Why is so? c) Water has pKa=15.74, and acetylene has pKa=25. Which is the stronger acid? Write down the structure of respective conjugate base for water and acetylene. Page 1 d) Sight along the C2>C1 bond, 2-methylpropane (isobutane) and (1) draw a Newman projection of the most stable conformation. (ii) draw a Newman projection of the least stable conformation. (iii) Since an H-Heclipsing interaction costs 4.0 kJ/mol and an H CH3 eclipsing interaction costs 6.0kJ/mol,what is the energy differences between the most and least stable conformations. e) Assign R or S configuration to the chirality center (where applicable) in each of the following structures. Which of the following structure is/are achiral? H OH HO H HO H H. II IV f) How many stereoisomers are possible for 3, 4 dibromo hexane? Show the structures of them in Wedge formula and assign R or S configuration to the chirality centers. And also comment on their optical activity.
Step by Step Solution
★★★★★
3.46 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
2 a Heterolytic cleavage is carried out in polar solvents by means of acids bases or ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


