Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 50.0 mL solution of 0.15 M HCI was used to dissolve a 1.45 g of antacid tablet. The 2.09 excess HCl was then
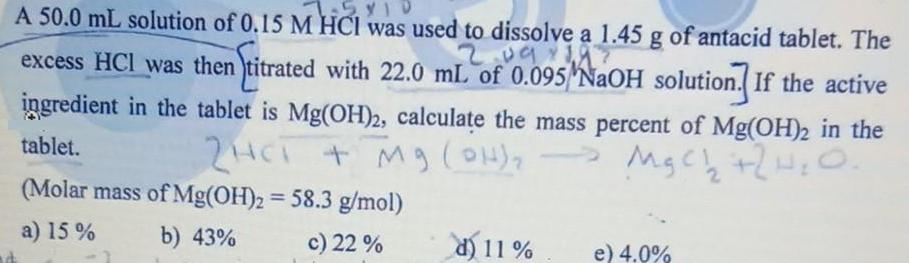
A 50.0 mL solution of 0.15 M HCI was used to dissolve a 1.45 g of antacid tablet. The 2.09 excess HCl was then )titrated with 22.0 mL of 0.095 NaOH solution. If the active ingredient in the tablet is Mg(OH)2, calculate the mass percent of Mg(OH)2 in the tablet. + Mg (OH) (Molar mass of Mg(OH)2 = 58.3 g/mol) %3D a) 15 % b) 43% c) 22 % d) 11 % e) 4.0%
Step by Step Solution
★★★★★
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636a45359fd1e_243118.pdf
180 KBs PDF File
636a45359fd1e_243118.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


