Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Close to 6 billion pounds of ethylene glycol (EG) were produced in 2007. It previously ranked as the twenty-sixth most produced chemical in the nation
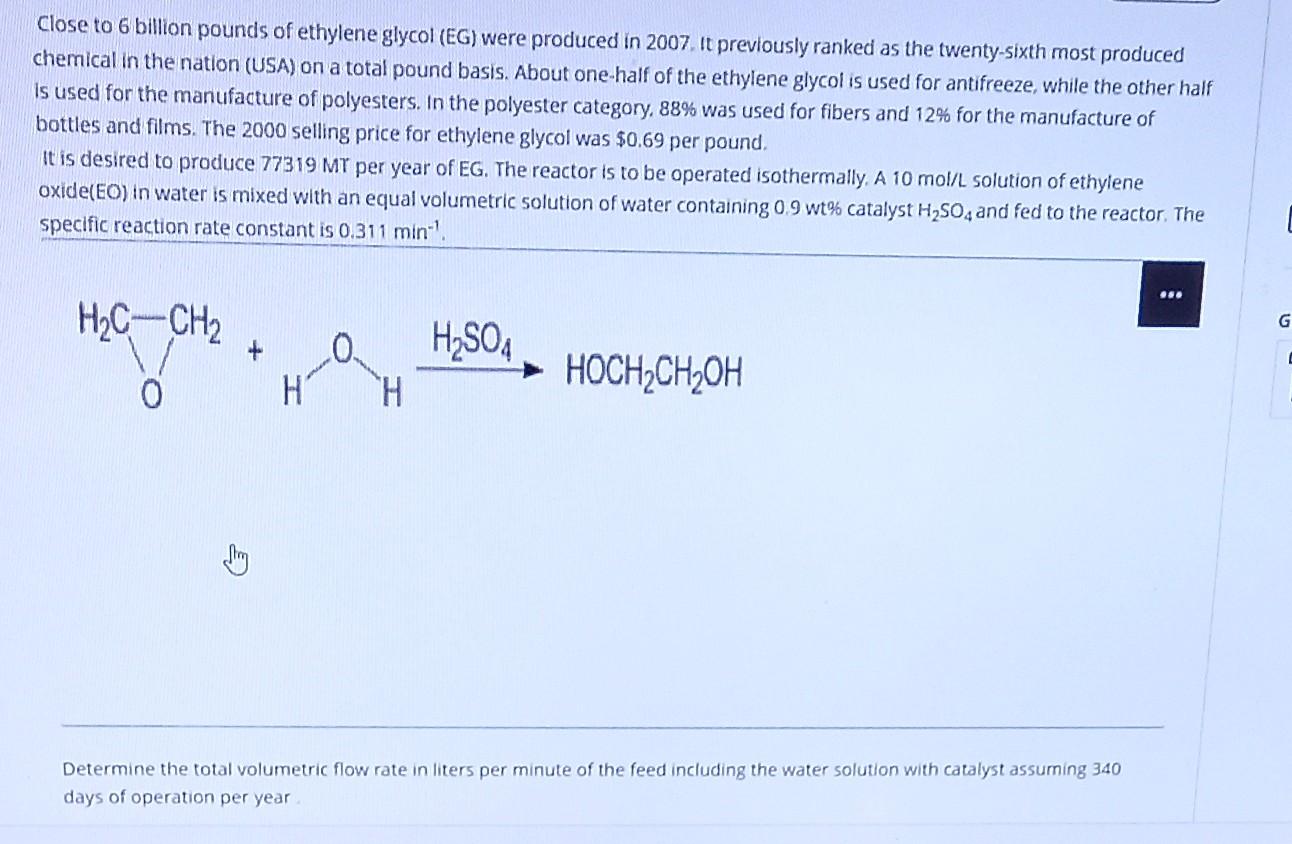
Close to 6 billion pounds of ethylene glycol (EG) were produced in 2007. It previously ranked as the twenty-sixth most produced chemical in the nation (USA) on a total pound basis. About one-half of the ethylene glycol is used for antifreeze, while the other half Is used for the manufacture of polyesters. In the polyester category, 88% was used for fibers and 12% for the manufacture of bottles and films. The 2000 selling price for ethylene glycol was $0.69 per pound, It is desired to produce 77319 MT per year of EG. The reactor is to be operated isothermally, A 10 mol/L solution of ethylene oxide(EO) in water is mixed with an equal volumetric solution of water containing 0.9 wt% catalyst H2SO4 and fed to the reactor. The specific reaction rate constant is 0.311 min ... H2C-CH2 G 0 H2SO4 + HOCH2CH2OH H H Determine the total volumetric flow rate in liters per minute of the feed including the water solution with catalyst assuming 340 days of operation per year
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


