Answered step by step
Verified Expert Solution
Question
1 Approved Answer
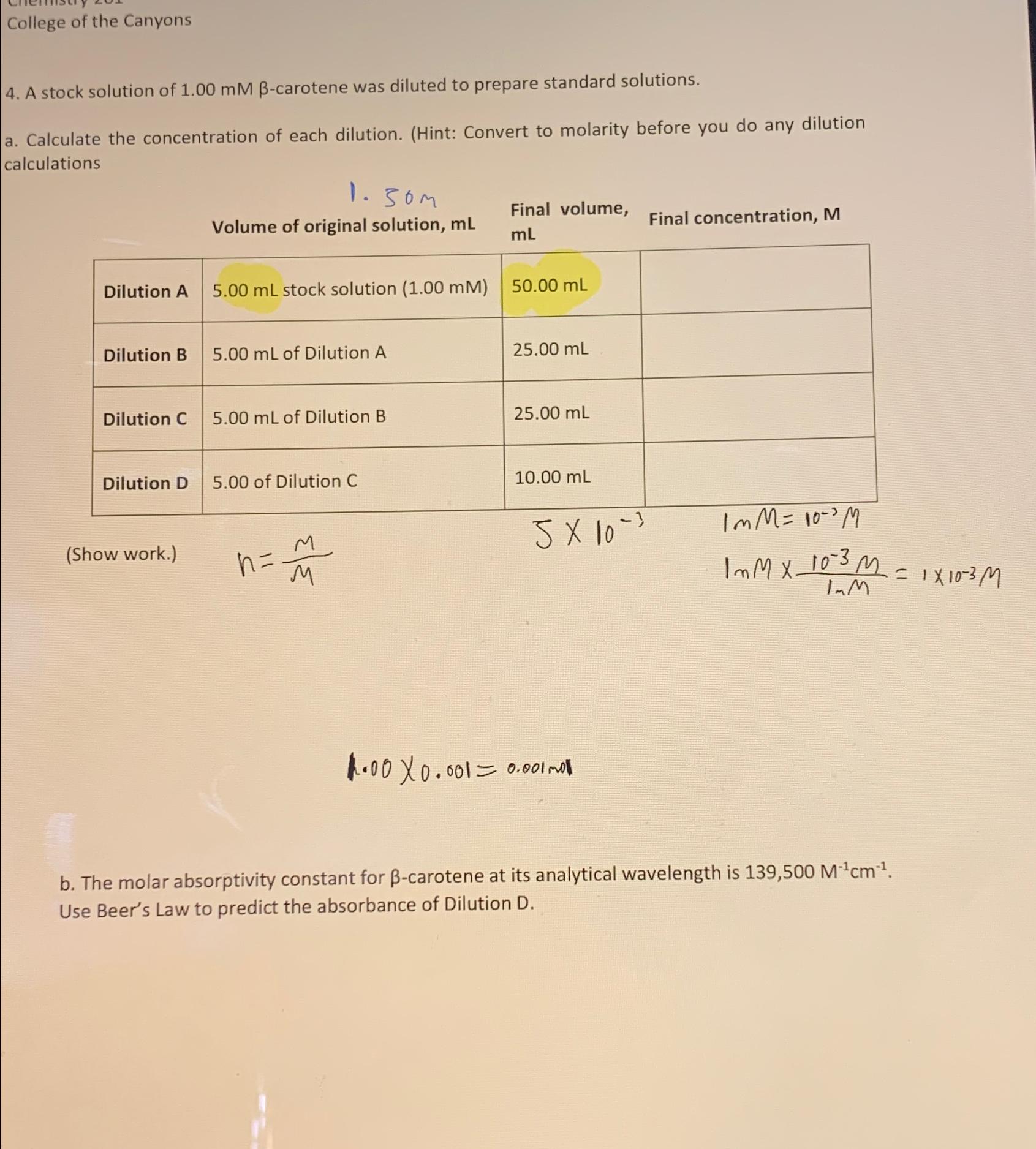
College of the Canyons 4 . A stock solution of 1 . 0 0 m M - carotene was diluted to prepare standard solutions. a
College of the Canyons
A stock solution of carotene was diluted to prepare standard solutions.
a Calculate the concentration of each dilution. Hint: Convert to molarity before you do any dilution calculations
Final volume,
Volume of original solution, Final concentration,
tableDilution A stock solution Dilution B of Dilution ADilution C of Dilution BDilution D of Dilution C
Show work.
mal
b The molar absorptivity constant for carotene at its analytical wavelength is Use Beer's Law to predict the absorbance of Dilution D
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


