Question
one two three four five six zero Complete the table below for the composition of hybrid orbitals. Atomic orbitals mixed Hybrid orbitals formed Unhybridized
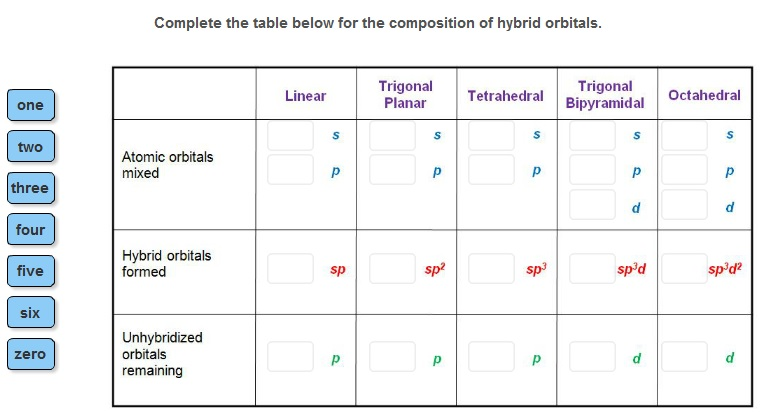
one two three four five six zero Complete the table below for the composition of hybrid orbitals. Atomic orbitals mixed Hybrid orbitals formed Unhybridized orbitals remaining Linear S sp Trigonal Planar S sp Tetrahedral $ sp Trigonal Bipyramidal S d spd d Octahedral $ d spd P
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Answer 1 Linear geometry One s orbital overlaps with one p orbital to form two sp hybrid orbitals Tw...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Horngrens Financial and Managerial Accounting
Authors: Tracie L. Nobles, Brenda L. Mattison, Ella Mae Matsumura
5th edition
9780133851281, 013385129x, 9780134077321, 133866297, 133851281, 9780133851298, 134077326, 978-0133866292
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



