Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Compound C can be made by reacting A with B in the following gas phase reaction A+3B2C The fresh feed contains 1% Ar as unreactive
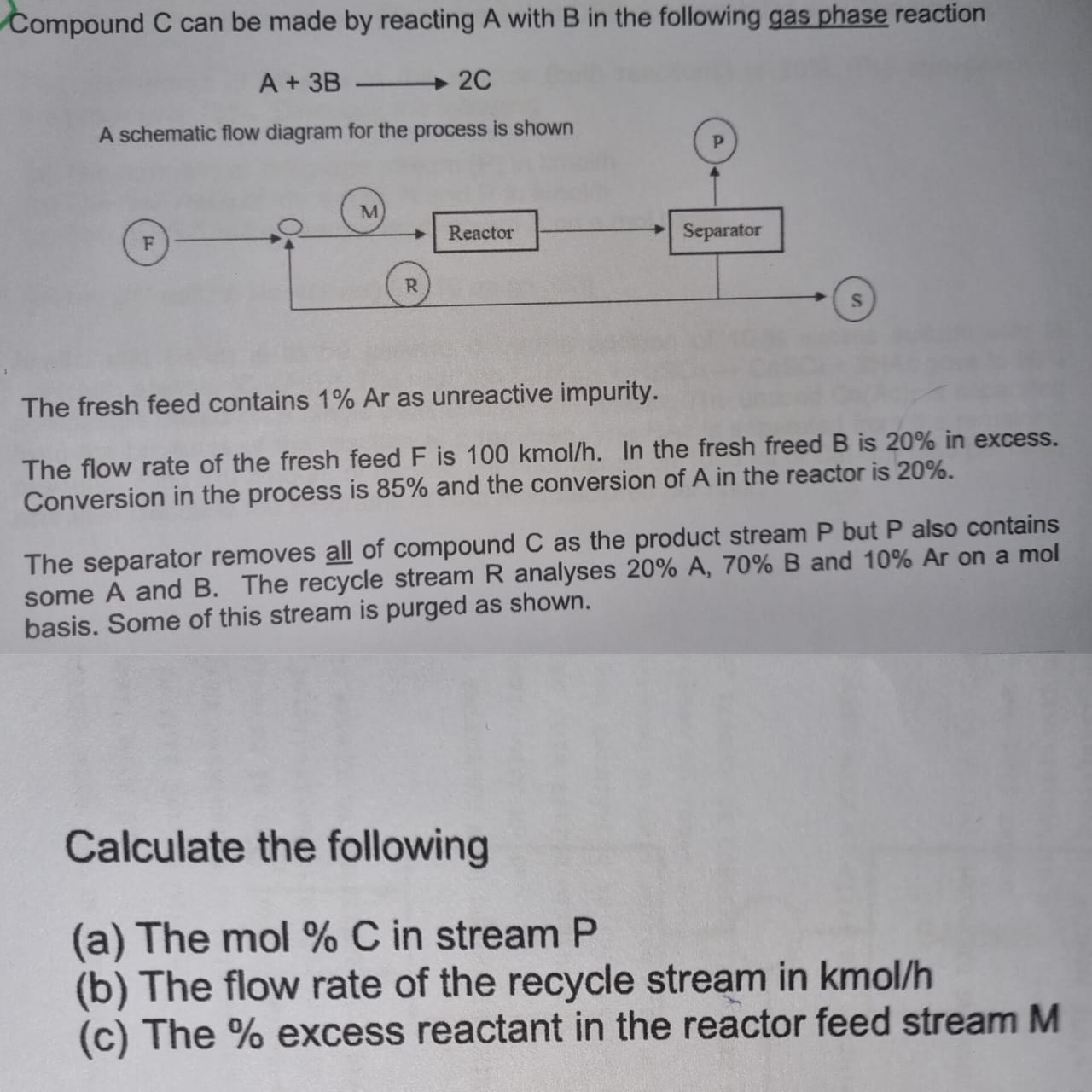 Compound C can be made by reacting A with B in the following gas phase reaction A+3B2C The fresh feed contains 1% Ar as unreactive impurity. The flow rate of the fresh feed F is 100kmol/h. In the fresh freed B is 20% in excess. Conversion in the process is 85% and the conversion of A in the reactor is 20%. The separator removes all of compound C as the product stream P but P also contains some A and B. The recycle stream R analyses 20%A,70%B and 10%Ar on a mol basis. Some of this stream is purged as shown. Calculate the following (a) The mol%C in stream P (b) The flow rate of the recycle stream in kmol/h (c) The \% excess reactant in the reactor feed stream M
Compound C can be made by reacting A with B in the following gas phase reaction A+3B2C The fresh feed contains 1% Ar as unreactive impurity. The flow rate of the fresh feed F is 100kmol/h. In the fresh freed B is 20% in excess. Conversion in the process is 85% and the conversion of A in the reactor is 20%. The separator removes all of compound C as the product stream P but P also contains some A and B. The recycle stream R analyses 20%A,70%B and 10%Ar on a mol basis. Some of this stream is purged as shown. Calculate the following (a) The mol%C in stream P (b) The flow rate of the recycle stream in kmol/h (c) The \% excess reactant in the reactor feed stream M Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


