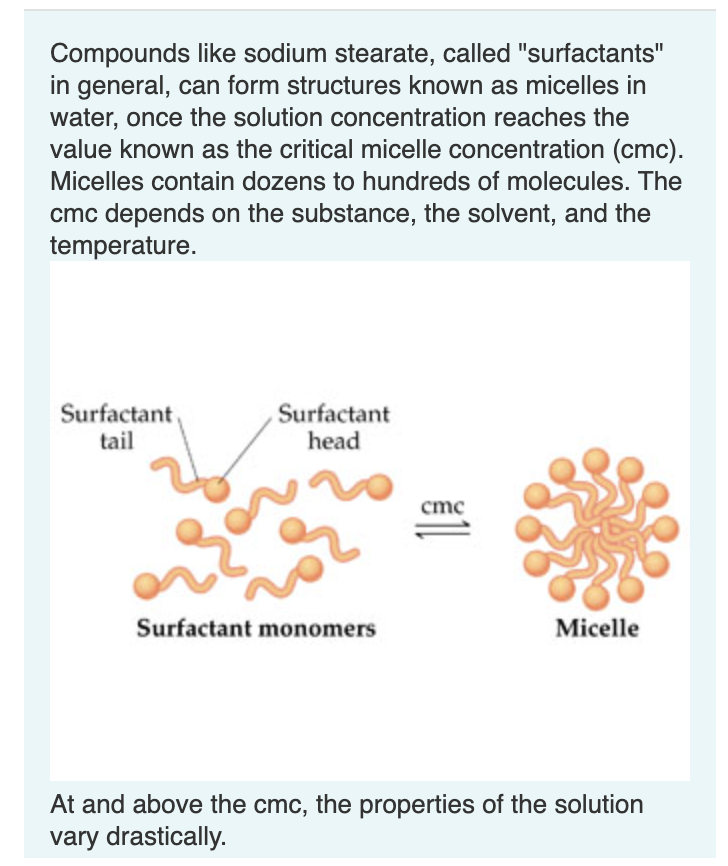
Compounds like sodium stearate, called "surfactants" in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature. At and above the cmc, the properties of the solution vary drastically. The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation. Drag the terms on the left to the appropriate blanks on the right to complete the sentences. True solutions light, colloids Below the critical micelle concentration, cmc, the mixture of solvent and surfactant is Above the cmc, the mixture is . The micelles are too to be perfectly mixed in the solvent. They are suspended in the solvent, resulting in that scatters light. The ionic conductivity of the solution dramatically changes at the cmc. Suggest an explanation. Drag the terms on the left to the appropriate blanks on the right to complete the sentences. Surfactant monomers are ; the "head" carries a charge. the cmc, each monomer is an independent particle. the cmc, many monomers aggregate into one micelle, drastically reducing the effective number of particles "in solution". (A micelle does have a greater charge than a monomer.) This dramatically changes the ionic conductivity. Chemists have developed fluorescent dyes that glow brightly only when the dye molecules are in a hydrophobic environment. Predict how the intensity of such fluorescence would relate to the concentration of sodium stearate as the sodium stearate concentration approaches and then increases past the Drag the terms on the left to the appropriate blanks on the right to complete the sentences. At low sodium stearate concentrations, the fluorescent intensity will be . As the surfactant concentration increases, fluorescent intensity gradually until the cmc is reached (assuming a few micelles form at concentrations cmc ). At the cmc, there is a large in fluorescent intensity. At concentrations greater than the cmc, fluorescent intensity remains and probably gradually










