Compute for the Relative Atomic Mass of the following isotopes: 1. Potassium has three stable isotopes, potassium-39, potassium-40, and potassium-41. Calculate the average atomic
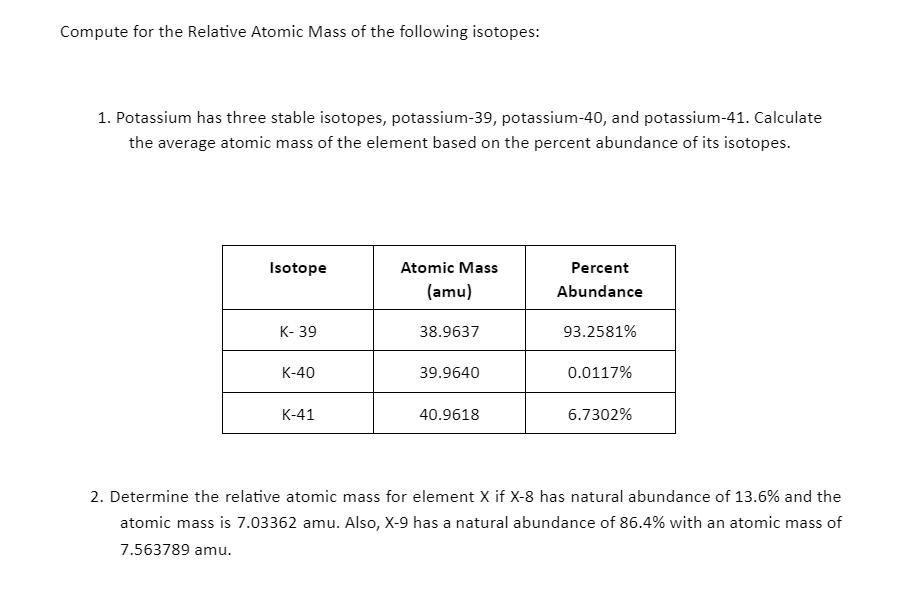
Compute for the Relative Atomic Mass of the following isotopes: 1. Potassium has three stable isotopes, potassium-39, potassium-40, and potassium-41. Calculate the average atomic mass of the element based on the percent abundance of its isotopes. Isotope K-39 K-40 K-41 Atomic Mass (amu) 38.9637 39.9640 40.9618 Percent Abundance 93.2581% 0.0117% 6.7302% 2. Determine the relative atomic mass for element X if X-8 has natural abundance of 13.6% and the atomic mass is 7.03362 amu. Also, X-9 has a natural abundance of 86.4% with an atomic mass of 7.563789 amu.
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
1 The average atomic mass of an element is calculated by taking the weighted average of the masses o...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


