Question
Consider a capillary tube of length L filled with a solid polymerized gel-type substance (Ca-alginate). One end of the tube is immersed in an infinitely
Consider a capillary tube of length L filled with a solid polymerized gel-type substance (Ca-alginate). One end of the tube is immersed in an infinitely large pool of liquid containing reactant A of concentration CAo . The other end of the capillary is sealed and contains coated catalyst that promotes the following chemical reaction
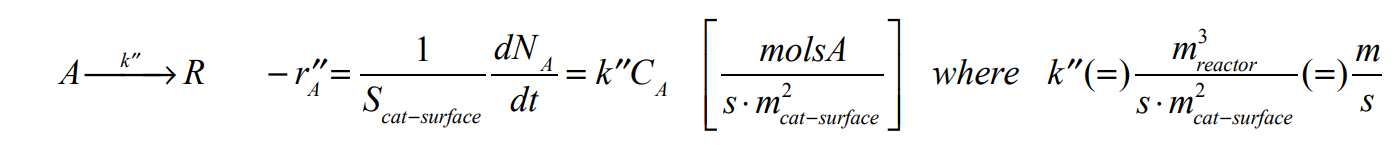
The reaction takes place only at the end of the capillary that contains the catalyst. Reaction does not take place within the gel itself.
Formulate differential equation and boundary conditions describing this process; (diffusion of reactant A through the gel and reaction at the end of the capillary). Solve analytically the differential equation for the unsteady state case. Make a graph of the unsteady state solution (show the concentration of reactant A along the capillary tube at different times starting at time t=0, and then after different time intervals t=t1, t=t2, . . .t=t.) Make sure you first define a realistic set of values for the parameters of your system: Lcap [m]; Di m2 / s ; dcap [m]; k mgel 3 / mcatalyst 2 s ; CAo mol / mgel 3 )
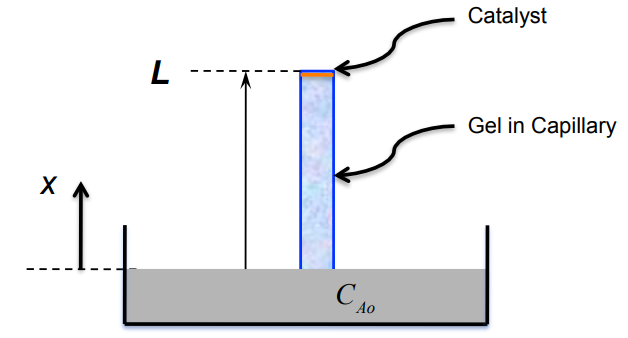
1 mols A Ak" R dN 4 = kCA -r"= S reactor m? m where k"(=) -(=) Sim S cat-surface dt 2 Sm cat-surface cat-surface Catalyst L Gel in Capillary XA
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


