Question
Consider a container with a frictionless piston that contains a given amount of an ideal gas. If the external pressure is kept constant, the piston
Consider a container with a frictionless piston that contains a given amount of an ideal gas. If the external pressure is kept constant, the piston will move up or down in response to a change in the internal pressure. The piston will move up if Pint > Pext and vice versa. The piston will stop moving when Pint = Pext (the system is equilibrated).
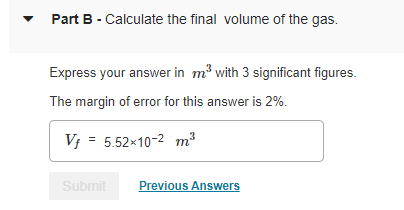
The container has 1.1 molmol of an ideal gas initially at 18 CC and the system is in equilibrium with an external pressure of 1 barbar . The gas is slowly heated to a final temperature of 330 CC . During this process, the piston is allowed to move if necessary to keep the system at equilibrium (that is, the internal pressure is allowed to match the external pressure at all times). The molar heat capacity at constant volume Cv,m of this ideal gas is 2.5 R. 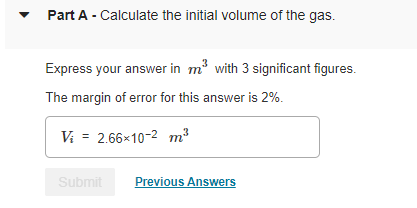
WHAT IS PART C, D, E? 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


