Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a continuously stirred tank with a cooling system. In the tank there is an exothermic chemical reaction. The reaction is unstable, which without cooling
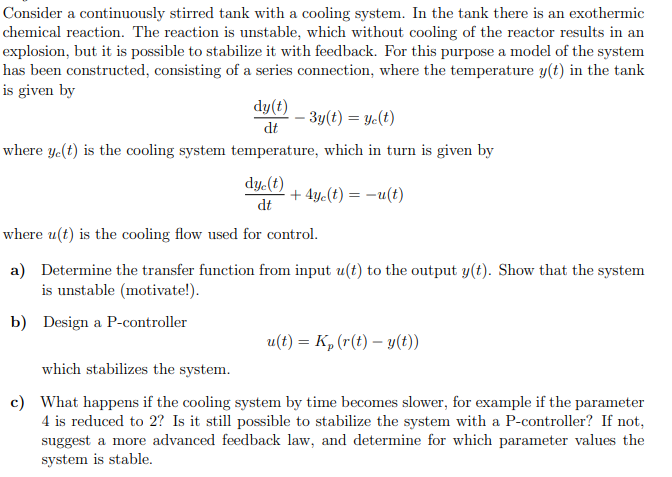 Consider a continuously stirred tank with a cooling system. In the tank there is an exothermic chemical reaction. The reaction is unstable, which without cooling of the reactor results in an explosion, but it is possible to stabilize it with feedback. For this purpose a model of the system has been constructed, consisting of a series connection, where the temperature y(t) in the tank is given by dtdy(t)3y(t)=yc(t) where yc(t) is the cooling system temperature, which in turn is given by dtdyc(t)+4yc(t)=u(t) where u(t) is the cooling flow used for control. a) Determine the transfer function from input u(t) to the output y(t). Show that the system is unstable (motivate!). b) Design a P-controller u(t)=Kp(r(t)y(t)) which stabilizes the system. c) What happens if the cooling system by time becomes slower, for example if the parameter 4 is reduced to 2 ? Is it still possible to stabilize the system with a P-controller? If not, suggest a more advanced feedback law, and determine for which parameter values the system is stable
Consider a continuously stirred tank with a cooling system. In the tank there is an exothermic chemical reaction. The reaction is unstable, which without cooling of the reactor results in an explosion, but it is possible to stabilize it with feedback. For this purpose a model of the system has been constructed, consisting of a series connection, where the temperature y(t) in the tank is given by dtdy(t)3y(t)=yc(t) where yc(t) is the cooling system temperature, which in turn is given by dtdyc(t)+4yc(t)=u(t) where u(t) is the cooling flow used for control. a) Determine the transfer function from input u(t) to the output y(t). Show that the system is unstable (motivate!). b) Design a P-controller u(t)=Kp(r(t)y(t)) which stabilizes the system. c) What happens if the cooling system by time becomes slower, for example if the parameter 4 is reduced to 2 ? Is it still possible to stabilize the system with a P-controller? If not, suggest a more advanced feedback law, and determine for which parameter values the system is stable Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


