Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a first-order irreversible reaction that occurs in a packed bed reactor where CA has units of mol/m. At a point in the reactor,
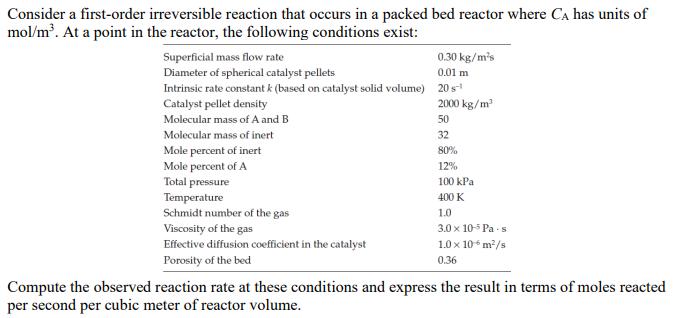
Consider a first-order irreversible reaction that occurs in a packed bed reactor where CA has units of mol/m. At a point in the reactor, the following conditions exist: Superficial mass flow rate Diameter of spherical catalyst pellets 0.30 kg/ms Intrinsic rate constant k (based on catalyst solid volume) 20s- Catalyst pellet density Molecular mass of A and B Molecular mass of inert 0.01 m 2000 kg/m 50 32 80% 12% Mole percent of inert Mole percent of A Total pressure Temperature Schmidt number of the gas Viscosity of the gas Effective diffusion coefficient in the catalyst Porosity of the bed 100 kPa 400 K 1.0 3.0 x 10-5 Pas 1.0 10 6 m/s 0.36 Compute the observed reaction rate at these conditions and express the result in terms of moles reacted per second per cubic meter of reactor volume.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


