Answered step by step
Verified Expert Solution
Question
1 Approved Answer
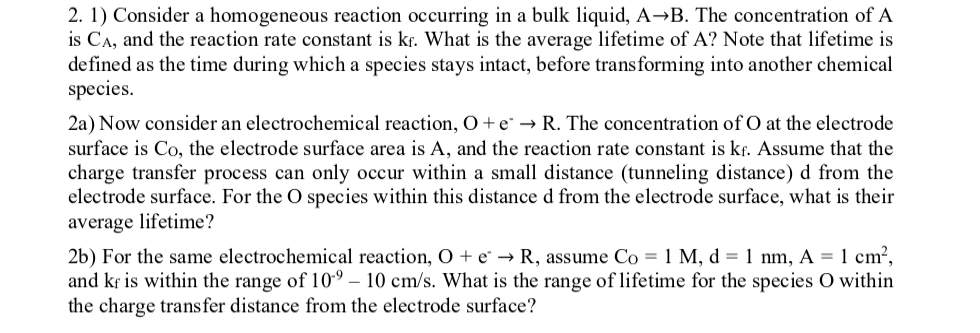
Consider a homogeneous reaction occurring in a bulk liquid, A B . The concentration of A is C A , and the reaction rate constant
Consider a homogeneous reaction occurring in a bulk liquid, The concentration of is and the reaction rate constant is What is the average lifetime of Note that lifetime is defined as the time during which a species stays intact, before transforming into another chemical species.
a Now consider an electrochemical reaction, The concentration of at the electrode surface is the electrode surface area is and the reaction rate constant is Assume that the charge transfer process can only occur within a small distance tunneling distance d from the electrode surface. For the species within this distance d from the electrode surface, what is their average lifetime?
b For the same electrochemical reaction, assume and is within the range of What is the range of lifetime for the species within the charge transfer distance from the electrode surface?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


