Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a rectangular-shaped gel tablet of thickness 0.652cm, width of 1.0cm and depth of 1.0 cm loaded with the drug Dramamine. The edges of the
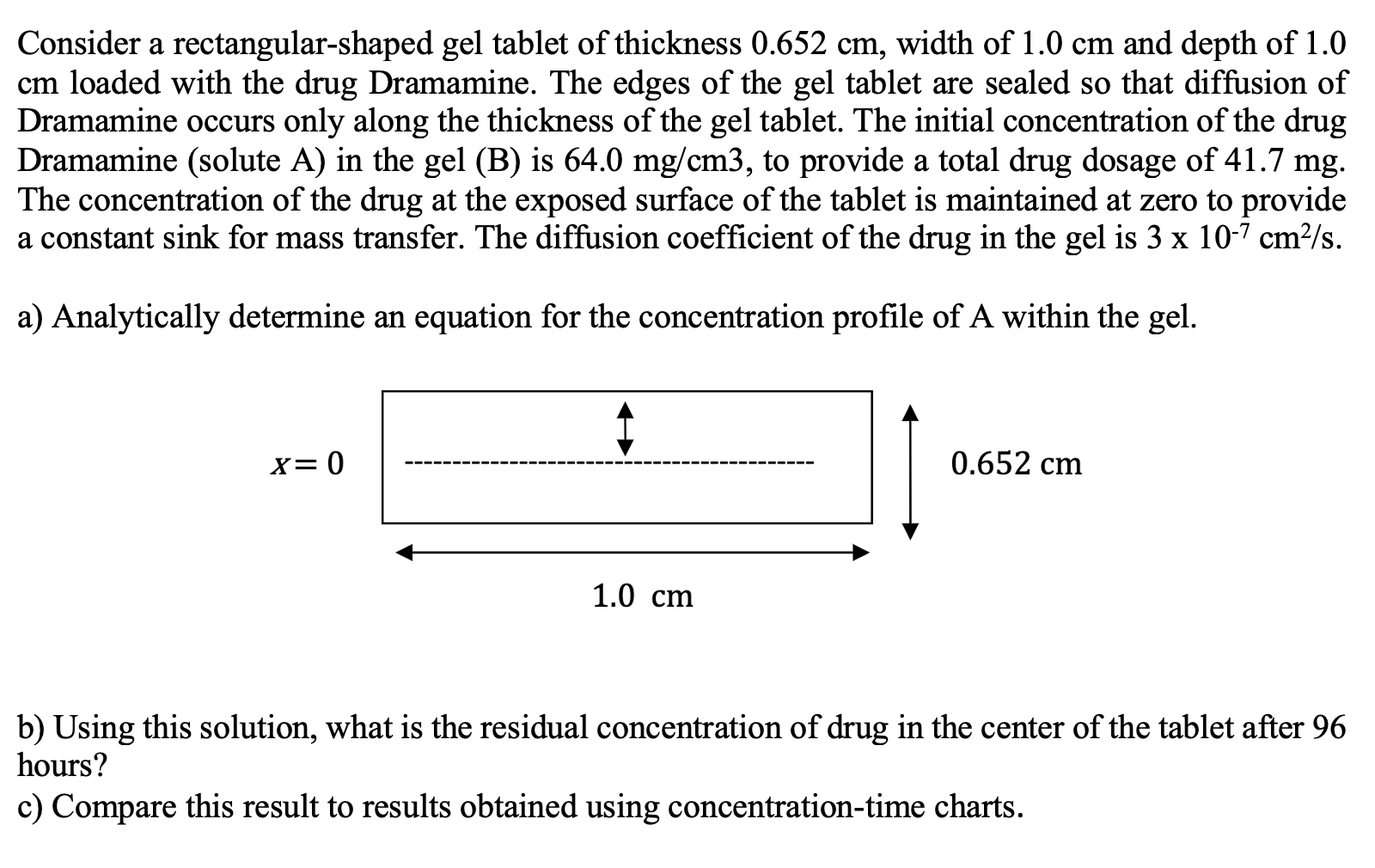 Consider a rectangular-shaped gel tablet of thickness 0.652cm, width of 1.0cm and depth of 1.0 cm loaded with the drug Dramamine. The edges of the gel tablet are sealed so that diffusion of Dramamine occurs only along the thickness of the gel tablet. The initial concentration of the drug Dramamine (solute A) in the gel (B) is 64.0mg/cm3, to provide a total drug dosage of 41.7mg. The concentration of the drug at the exposed surface of the tablet is maintained at zero to provide a constant sink for mass transfer. The diffusion coefficient of the drug in the gel is 3107cm2/s. a) Analytically determine an equation for the concentration profile of A within the gel. b) Using this solution, what is the residual concentration of drug in the center of the tablet after 96 hours? c) Compare this result to results obtained using concentration-time charts. Consider a rectangular-shaped gel tablet of thickness 0.652cm, width of 1.0cm and depth of 1.0 cm loaded with the drug Dramamine. The edges of the gel tablet are sealed so that diffusion of Dramamine occurs only along the thickness of the gel tablet. The initial concentration of the drug Dramamine (solute A) in the gel (B) is 64.0mg/cm3, to provide a total drug dosage of 41.7mg. The concentration of the drug at the exposed surface of the tablet is maintained at zero to provide a constant sink for mass transfer. The diffusion coefficient of the drug in the gel is 3107cm2/s. a) Analytically determine an equation for the concentration profile of A within the gel. b) Using this solution, what is the residual concentration of drug in the center of the tablet after 96 hours? c) Compare this result to results obtained using concentration-time charts
Consider a rectangular-shaped gel tablet of thickness 0.652cm, width of 1.0cm and depth of 1.0 cm loaded with the drug Dramamine. The edges of the gel tablet are sealed so that diffusion of Dramamine occurs only along the thickness of the gel tablet. The initial concentration of the drug Dramamine (solute A) in the gel (B) is 64.0mg/cm3, to provide a total drug dosage of 41.7mg. The concentration of the drug at the exposed surface of the tablet is maintained at zero to provide a constant sink for mass transfer. The diffusion coefficient of the drug in the gel is 3107cm2/s. a) Analytically determine an equation for the concentration profile of A within the gel. b) Using this solution, what is the residual concentration of drug in the center of the tablet after 96 hours? c) Compare this result to results obtained using concentration-time charts. Consider a rectangular-shaped gel tablet of thickness 0.652cm, width of 1.0cm and depth of 1.0 cm loaded with the drug Dramamine. The edges of the gel tablet are sealed so that diffusion of Dramamine occurs only along the thickness of the gel tablet. The initial concentration of the drug Dramamine (solute A) in the gel (B) is 64.0mg/cm3, to provide a total drug dosage of 41.7mg. The concentration of the drug at the exposed surface of the tablet is maintained at zero to provide a constant sink for mass transfer. The diffusion coefficient of the drug in the gel is 3107cm2/s. a) Analytically determine an equation for the concentration profile of A within the gel. b) Using this solution, what is the residual concentration of drug in the center of the tablet after 96 hours? c) Compare this result to results obtained using concentration-time charts Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


