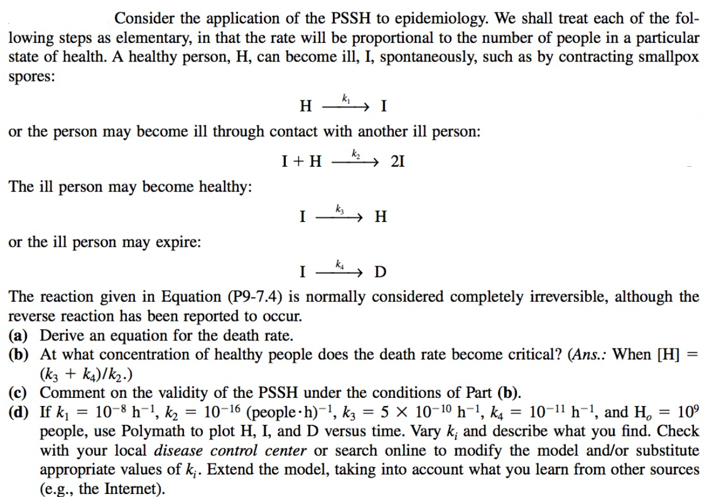
Consider the application of the PSSH to epidemiology. We shall treat each of the following steps as elementary, in that the rate will be proportional to the number of people in a particular state of health. A healthy person, H, can become ill, I, spontaneously, such as by contracting smallpox spores: Hk1I or the person may become ill through contact with another ill person: I+Hk22I The ill person may become healthy: Ik3H or the ill person may expire: Ik4D The reaction given in Equation (P9-7.4) is normally considered completely irreversible, although the reverse reaction has been reported to occur. (a) Derive an equation for the death rate. (b) At what concentration of healthy people does the death rate become critical? (Ans.: When [H]= (k3+k4)/k2) (c) Comment on the validity of the PSSH under the conditions of Part (b). (d) If k1=108h1,k2=1016 (people h)1,k3=51010h1,k4=1011h1, and Ho=109 people, use Polymath to plot H, I, and D versus time. Vary ki and describe what you find. Check with your local disease control center or search online to modify the model and/or substitute appropriate values of ki. Extend the model, taking into account what you learn from other sources (e.g. the Internet). Consider the application of the PSSH to epidemiology. We shall treat each of the following steps as elementary, in that the rate will be proportional to the number of people in a particular state of health. A healthy person, H, can become ill, I, spontaneously, such as by contracting smallpox spores: Hk1I or the person may become ill through contact with another ill person: I+Hk22I The ill person may become healthy: Ik3H or the ill person may expire: Ik4D The reaction given in Equation (P9-7.4) is normally considered completely irreversible, although the reverse reaction has been reported to occur. (a) Derive an equation for the death rate. (b) At what concentration of healthy people does the death rate become critical? (Ans.: When [H]= (k3+k4)/k2) (c) Comment on the validity of the PSSH under the conditions of Part (b). (d) If k1=108h1,k2=1016 (people h)1,k3=51010h1,k4=1011h1, and Ho=109 people, use Polymath to plot H, I, and D versus time. Vary ki and describe what you find. Check with your local disease control center or search online to modify the model and/or substitute appropriate values of ki. Extend the model, taking into account what you learn from other sources (e.g. the Internet)







